1. Background
Early hospital discharge is the most common cause of rehospitalization for jaundice (1) and is a risk factor for kernicterus (2, 3). Kernicterus (bilirubin encephalopathy) results from the deposition of unconjugated (indirect reacting) bilirubin in the basal ganglia and brainstem nuclei. It is also important to know that Kernicterus is an acquired metabolic encephalopathy in the neonatal period (4). Early discharge of healthy term newborns within the first 48 hours of life is a common practice due to medical, social, and economic reasons (5). Owing to the early discharge as well as the development of hyperbilirubinemia in newborns, there is a lot of discussions and controversies in this regard (6). Consequently, recognition, follow up, and treatment of jaundice at early stages is difficult due to such discharge policies. We should be aware that kernicterus and severe jaundice can occur in some full-term healthy newborns that are discharged soon without clear, early findings of hemolysis (7). The American Academy of Pediatrics (AAP) suggests that newborns discharged within 48 hours should have a follow–up visit after 48 to 72 hours for any hyperbilirubinemia and other complications (8). This suggestion is impractical in Iran because of limited follow-up facilities as well as long distances to existing facilities.
Although our understanding concerning the physiological features of bilirubin and the mechanisms of bilirubin neurotoxicity has improved, our ability to make predictions to know which infants are at greatest risk remains limited. Predicting jaundice can be considered as an interesting tool to choose babies at risk of neonatal hyperbilirubinemia (9). It is difficult to make predictions concerning which infants are at increased risk for significant and relatively late hyperbilirubinemia. There is an obvious need to apply follow-up programs or to develop predictive guidelines that will enable physicians to predict or to identify which early discharged newborns will develop hyperbilirubinemia (10). Therefore, predicting the risk of jaundice for implementing the early treatment and consequently minimizing the risk of bilirubin-dependent brain damage is of salient importance.
2. Objectives
The objective of the present study was to find out the critical value of serum bilirubin in the cord blood in order to predict later development of hyperbilirubinemia in healthy term newborns up to their third day of life.
3. Methods
This prospective, cohort study was undertaken in the department of pediatrics at Afzalipour Hospital in Kerman, Iran. This study, conducted over a period of 9 months, was approved by the Ethics Committee of Kerman University of Medical Sciences. A total of 300 healthy newborns were included in the study. The inclusion criteria were: sequentially born term babies (gestational age > 37 weeks) from any mode of delivery, both genders, birth weight above 2500 g, Apgar score above 7 at first and fifth minutes of life, and without ABO and Rh incompatibility. The exclusion criteria were: babies who had major congenital malformations, Glucose-6-phosphate dehydrogenase deficiency, or birth asphyxia, NICU admitted babies due to severe illness or sepsis, cephalohematoma, prolonged rupture of membrane (more than 18 hours) and bruising. The gestational age was determined based on the findings of first-trimester ultrasound (when available) or on account of the date of the last menstrual period. This was confirmed with the new Ballard score within 24 hours after birth. Two ml of cord blood were collected during delivery in two plain vials and were sent to the clinical laboratory of Afzalipour Hospital in order to determine the blood group and estimate the serum total, unconjugated, and conjugated bilirubin levels using a colorimetric method (Selectra XL, The Netherlands). Parents of all newborns were contacted and consent was obtained. The maternal blood group was obtained from medical records. The relevant history of mother and baby was taken and thorough physical and clinical examination of all neonates was performed. All recruited neonates were assessed clinically for the development of jaundice and other illnesses, every day, from birth until discharge by members of the medical team. Whenever necessary, further assessment for bilirubin level was done. The subjects were asked to return for follow-up after discharge at 72 hours of age or younger if parents found their baby icteric. The follow-up included clinical assessment and checking of bilirubin by transcutaneous bilirubinometry (TCB). TCB is a non-invasive method to measure bilirubin. It functions by directing white light into the skin and measures the intensity of the specific wavelengths returned. TCB measurement was done by JM-103 Jaundice Meter, Draeger medical AG & Co, Lubeck, Germany. This measurement provides moderately accurate estimates of TB in term newborn infants with different races and ethnicities (11). Hyperbilirubinemia, which required therapy, was defined as TCB level of 15 mg/dL or more according to the percentile-based hour-specific transcutaneous bilirubin nomogram (12). If TCB was equal or more than 15 mg/dL, neonates were subjected to a serum venous bilirubin sample collection and appropriate intervention and treatment were then applied. The need for phototherapy was determined according to AAP2004 guidelines (11). Neonates who failed to return for follow-up were excluded from the study. We looked mainly for hyperbilirubinemia which needed phototherapy or exchange transfusion in healthy term newborns. Data were processed and analyzed using SPSS software for Windows version 20. Specificity, sensitivity, as well as negative and positive predictive value of four cut-points of cord bilirubin were obtained and the likelihood ratios of the test were calculated. Chi-square test, t-test and ROC curve were used whenever appropriate. P value < 0.05 was considered as statistically significant.
4. Results
Of 300 infants who were enrolled into the study, 62 were excluded as they did not return or were not followed up. In addition, those who had a bad general condition (sepsis, respiratory distress, or heart failure) were excluded. All included newborns were from Kerman or nearby districts for easy referral to the hospital. A total of 238 (113 boys and 125 girls) healthy term newborns were inducted into the study. Of those, 112 (47%) were born by vaginal delivery and 126 (53%) by cesarian section. The predominant blood type among mothers and neonates was type O. Table 1 depicts the baseline characteristics of cases that developed and did not develop hyperbilirubinemia.
| Variable | Hyperbilirubinemia (TCB ≥ 15 mg/dL) at 72 Hours of Life | No Hyperbilirubinemia (TCB ≤ 15 mg/dL) at 72 Hours of Life | P Value |
|---|---|---|---|
| Gender, male/female | 16/11 | 97/114 | 0.193 |
| Delivery, CS/NVD | 13/14 | 114/98 | 0.581 |
| Cord bilirubin (mg/dL), mean ± SD | 3.11 ± 0.72 | 2.71 ± 0.75 | 0.010 |
| TCB at 72 hours of life (mg/dL), mean ± SD | 16.78 ± 1.12 | 10.56 ± 2.87 | 0.001 |
The studied infants were categorized into two groups based on transcutaneous bilirubin level at 72 hours of age: 211 (88.6%) neonates in group A who did not develop hyperbilirubinemia (TCB < 15 mg/dL), and 27 (11.4%) in group B who had hyperbilirubinemia (TCB ≥ 15 mg/dL). In group B, newborns, according to the hour-specific normogram, were admitted for appropriate intervention and treatment. All neonates in group B required phototherapy and none required exchange transfusion. There were no significant differences between groups A and B in terms of different factors that may be associated with the risk of hyperbilirubinemia such as gender (P = 0.19) and type of delivery (P = 0.581). The mean (± SD) of UCSB level within group B (3.11 ± 0.72 mg/dL) was significantly (P < 0.010) higher in comparison to that within group A (2.71 ± 0.75 mg/dL). Table 1 shows the baseline characteristics of cases that developed and did not develop hyperbilirubinemia. This table demonstrates that the mean (± SD) of TCB level among the two groups was significantly different at 72 hours (16.78 ± 1.12 in group B vs 10.56 ± 2.87 in group A) (P < 0.001). Table 2 shows the relationship of serum bilirubin and cord bilirubin at 72 hours.
aValues are expressed as No. (%).
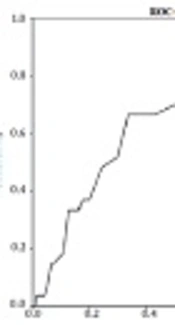
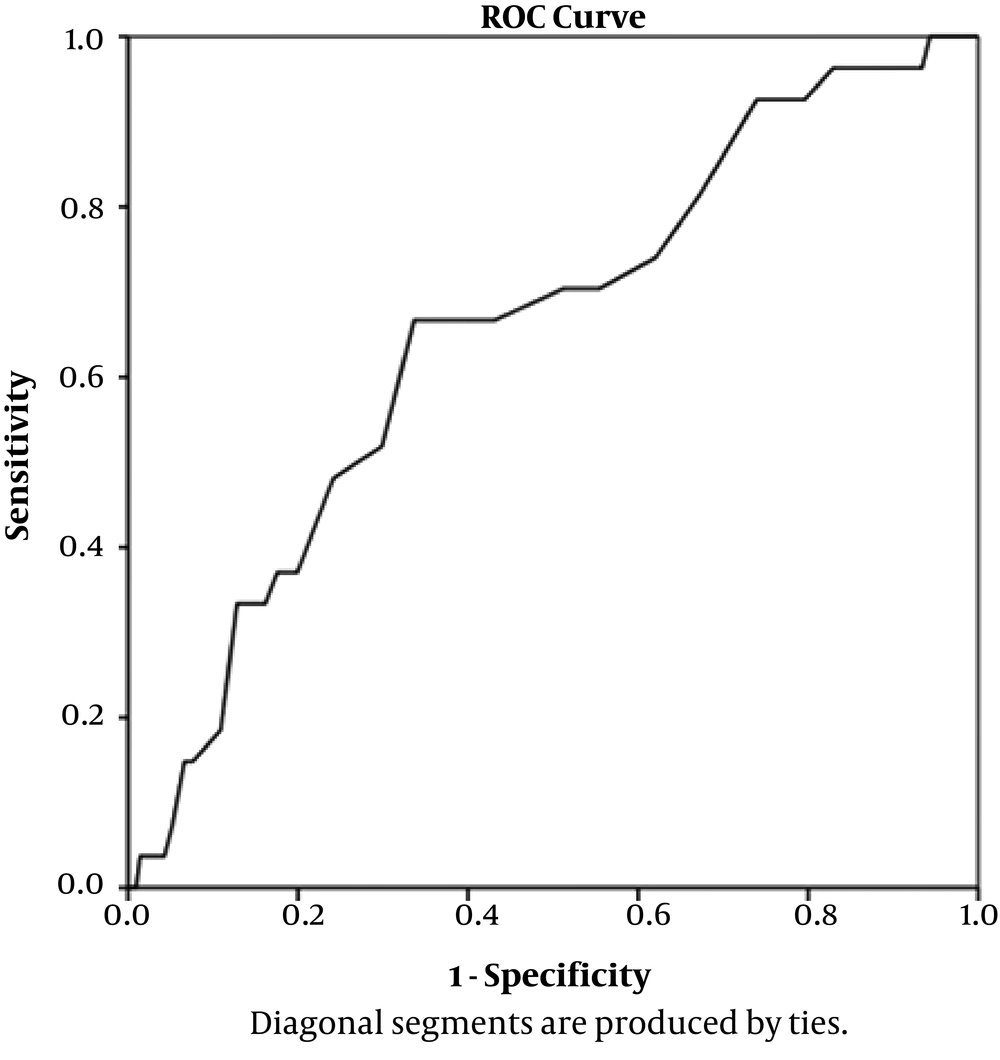
Of 182 newborns who had a UCSB level of ≥ 2.25 mg/dL, 27 (11.4%) developed hyperbilirubinemia which required treatment at 72 hours of life, whereas only two babies (7.2%) who had UCSB < 2.25 developed jaundice at 72 hours of age (Table 2). To identify a cut-off point of predicting a large number of babies at low risk of hyperbilirubinemia, an ROC curve was drawn (Figure 1).
The ideal cut-off was 2.25 mg/dL as just two babies (7.2%) below this level developed hyperbilirubinemia and 92.8% of jaundiced babies had UCSB levels above or equal to 2.25 mg/dl (Table 3). The different cut-off points for UCSB levels and the specificity, sensitivity, as well as positive predictive value and negative predictive value for each cut-off point is depicted in Table 3.
| Cut Off Value | |||
|---|---|---|---|
| 2.25 mg/dL | 2.55 mg/dL | 2.99 mg/dL | |
| Sensitivity | 92.6% | 70% | 66% |
| Specificity | 27% | 45% | 66% |
| Positive predictive value | 67% | 65% | 75% |
| Negative predictive value | 69% | 50% | 56% |
| True positive | 55% | 42% | 39% |
| False positive | 29% | 22% | 13.6% |
Based on ROC curve analysis (Figure 1), a mean cord bilirubin level of 2.25 mg/dL had the highest sensitivity (92.6%) to predict the neonates who would develop hyperbilirubinemia. At this critical mean cord bilirubin level, the positive predictive value was good (66%), and negative predictive value was 69%. At UCSB cut-off level of 2.99 mg/dL, the sensitivity and specificity was the same (66%).
5. Discussion
There is concern about the possibility of kernicterus and an increasing incidence of severe hyperbilirubinemia even in healthy term newborns with no apparent hemolysis. The problem is that predicting, recognizing, following up, and the early treatment of jaundice is difficult due to early discharge from hospital and lack of compliance by poor parents. Also, some of them might not attend for follow up and might be missed or uncontrolled. There are conflicting reports about the usefulness of measuring bilirubin level in cord blood in order to predict hyperbilirubinemia in newborns. Nevertheless, most agree about the value of measuring this parameter (6, 10, 12-14). Thus we can implement this simple bilirubin predicting method before bilirubin level reaches critical levels.
Our study was undertaken to determine prospectively the critical cord serum bilirubin level in order to predict hyperbilirubinemia in healthy term newborns. A cord bilirubin level ≥ 2.25 mg/dL predicts development of hyperbilirubinemia (define as TCB ≥ 15 mg/dL) with sensitivity of 92.6% at 72 hours of age. In the present study, we excluded many newborns for several reasons, including ABO/RH incompatibility, G6PD deficiency; preterm, late preterm, etc; because many studies (6, 15, 16) have shown these neonates are more likely to develop significant jaundice more than term healthy infants.
The mean total cord bilirubin at birth and TCB at 72 hours of age, had no statistically significant relation to gender and the type of delivery, and consistent with the results of some studies (10, 15, 17, 18). Two studies (19, 20) found that UCSB level was significantly different between the sexes. We assume that the difference may be the result of G6PD enzyme deficiency among males as it is an X-linked recessive disorder that was excluded from our study, and they did not exclude these types of babies. The incidence of jaundice is dependent upon ethnic makeup of the population, regional variations, etc. Our study showed 11.4% of newborns had hyperbilirubinemia and needed phototherapy. These results are not consistent with studies which showed that hyperbilirubinemia occurred in 6% of infants (10, 13, 20). This may be due to the fact that they did not follow neonates till the third day of life. On the other hand, Bernaldo and Segre (6) and Zeitoun et al. (15) found that the incidence of jaundice in newborns undergoing phototherapy was significantly higher (19.86% and 29.8% respectively). This could be explained thus that neonates with ABO incompatibility and G6PD deficiency were included in their study.
The mean of UCSB level for babies who received phototherapy was 3.11 ± 0.72 mg/dL which is significantly higher than those who did not receive it which was 2.71 ± 0.75 mg/dL. Similarly, Bernaldo and Segre (6), Zeitoun et al. (15) and Taksande et al. (10) found that phototherapy was significantly associated with higher levels of UCSB for infants who received it. Rostami et al. (17), demonstrated that UCSB level > 3 mg/dL cannot identify newborns with significant jaundice. In comparison to these results, the present study showed that 92.6% of infants who required phototherapy had UCSB level ≥ 2. 25 mg/dL, this is in line with other studies (6, 15). Satrya et al. (19) showed that UCSB level > 2.54 had high specificity and sensitivity. Zeitoun et al. (15) also found that UCSB level > 2.15 mg/dL had the highest sensitivity and at this critical cord bilirubin level, negative predictive value was very high. However, in our study, UCSB level < 2.25 mg/dL did not completely exclude the development of significant jaundice; 7.2% of newborns with UCSB < 2.25 mg/dL developed jaundice. Based on our findings, with a critical cutoff level of 2.25 mg, UCSB has very high sensitivity which means UCSB level can be used as a reliable screening test to diagnose healthy term infants at risk of hyperbilirubinemia.
Measurement of UCSB level can be used as a screening tool for the development of jaundice requiring intervention therapy. Neonates with UCSB level ≥ 2.25 mg/dL will need a close follow-up to be checked for the development of jaundice, whereas neonates with UCSB level < 2.25 mg/dL are probably safe for early discharge. We recommend that routine estimation of UCSB level be emphasized in all institutional deliveries to predict the subsequent development of significant jaundice in healthy term newborns.