1. Background
Duchenne-type muscular dystrophy (DMD) is an X-linked recessive disease that primarily affects the skeletal and cardiac muscles. DMD is the most common form of hereditary neuromuscular disorders that results from a dystrophin gene mutation which is located at Xp21.1. Dystrophin is the cytoskeletal protein that provides a mechanical integrity of the sarcolemma. Dystrophin deficiency causes mechanical weakening of the sarcolemma resulting in inappropriate calcium influx from loss of membrane integrity. The cascade of calcium influx activates proteases that lead to death of the cardiomyocytes. Degeneration begins at the cellular level, results in a cardiac fibrosis and ultimately formation of dilated cardiomyopathy. The main feature of the disease, as a result of skeletal muscle involvement is progressive muscle weakness (1-4).
Symptoms usually appear in male children before age 6 and may be visible in early infancy. In the later stage, progressive cardiac involvement and muscle degeneration can occur. Cardiac muscle degeneration is associated with fibrous tissue replacement with fatty infiltration. Degenerative changes in the myocardium can cause conduction abnormalities besides dilated cardiomyopathy phenotype (5-7). Myocardial degeneration that leads a maladaptive remodelling, results in cardiac diastolic and systolic dysfunction. This may induce cardiac failure and fatal arrhythmias (8). Cardiac symptoms usually become noticeable after the age of 10 years and increase in incidence with age (7).
Although recent studies suggested that some genotypes lead to develop cardiac failure in early period of the disease, this is not exactly proven (9-11). Echocardiography is usually first diagnostic tool for the diagnosis of cardiac involvement. However standard echocardiographic methods may fail to detect early changes of myocardial involvement. Tissue Doppler imaging is more useful in the demonstration of myocardial tissue abnormalities before the development of demonstrable systolic dysfunction (12, 13). This study was undertaken to characterize cardiac morphological and functional changes by echocardiography that are present in the early and late stages of DMD.
2. Methods
2.1. Patient Population
This study included all patients diagnosed as DMD who were on follow-up of the pedriatic cardiology department of the Istanbul University, Istanbul Medical Faculty. The final diagnosis of DMD was made on the basis of established criteria including genetic analysis, muscular biopsy, or typical clinical findings (neurologic signs, increased creatine phosphokinase levels, and typical calf hypertrophy) (14). Female carriers and patients with Becker muscular dystrophy were excluded.
2.2. Study and Control Groups Included in the Study
Study group was diveded into two subgroups as DMD patients with systolic impairment group Ia (n = 32) and those without systolic impairement Group Ib (n = 30). Systolic impairment group consisted of patients whose ejection fraction values were under 60 ± 5%. We wanted to observe if there was any difference between the patients according to their measured data. Our statisticians suggested to group patients according to their EF values. Because the patients had clustered around two values of EF 55 and 65 ranges, we decided to take 1 standard deviation above or below the mean range 60 ± 5% for ejection fraction (15). Control group consisted of 62 age- and gender-matched healthy children. Control subjects were obtained among children investigated for physiological cardiac murmur. The study protocol was approved by the Hospital Ethics Commitee, and written informed consent was obtained.
2.3. Echocardiography
Transthoracic echocardiography by 2-D, Doppler and M-mode was performed with an Vivid-3 echocardiography device (General Electric, USA) with a 3 MHz transducer. All patients were examined according to the recommendations of American society of echocardiography (16). Each echocardiographic measurement was taken at least three times. Left ventricular end diastolic diameter (LVEDd), left ventricular end systolic diameter (LVSd), ejection fraction (EF) and fractional shortening (FS) were measured by M-mode echocardiography. According to M-mode measurements left ventricular volumes were obtained by Teicholz formula (17).
Left ventricular mass (LV mass) was also estimated by M-mode measurements. For measurement of the components of LV mass including LVEDd, posterior wall thickness (LVPWd) and interventricular septal thickness (IVSd) the following formula was used: 0.8 × [1.04 × {(LVEDd + LVPWd + IVSd)3 - (LVEDd)3}] + 0.6g. We also calculated LV mass index (LV massi) by dividing the LV Mass to body surface area (BSA) (LV mass/ BSA (g/m2) (18).
Relative wall thickness (RWT) enabled to further classification of increase in LV mass as either concentric hypertrophy (RWT > 0.42) or eccentric hypertrophy (RWT ≤ 0.42). Relative wall thickness of the LV was stated as the ratio of twice the posterior wall thickness at end diastole to the end diastolic ventricular dimension (2 × LVPWd/LVEDd). The echocardiographic characteristics according to LV mass and RWT measurements determined left ventricular geometry pattern (19).
Mitral inflow patterns were recorded from the apical four-chamber view with the pulsed wave Doppler. Early (E) and late (A) transmitral inflow velocities, the ratio of early-to-late peak velocities (E/A) were recorded. Left ventricular myocardial diastolic properties were assessed using tissue Doppler imaging. Tissue Doppler measurements were performed in the apical four-chamber view. A sample volume was placed at the lateral corner of the mitral annulus for measurements of the peak early (E’) and late diastolic (A’) annular velocities. Cardiac diastolic function has passive and active processes. Ventricular relaxation is the active phase and determined by the ratio of E wave to A wave and ratio E’ to A’. The passive phase is the ventricular compliance that is determined by the ratio of E/E’ (20).
The key variables recommended for assessment of LV diastolic function grade include mitral flow velocities, mitral annular e’ velocity and E/E’ ratio (21).
We also measured pressure half time (PHT) and mitral valve area from the transmitral Doppler recordings. PHT is obtained by tracing the deceleration slope of the E-wave on Doppler spectral display of transmitral flow. Mitral valve area was determined by the formula 220/PTH (22, 23).
2.4. Statistical Methods
The statistical analysis was performed using the statistical package for social sciences version 15 (SPSS Inc, Chicago, IL, USA). All continuous variables were tested for normal distribution by Kolmogorov-Smirnov test. Baseline hemodynamic characteristics were compared between patients and controls using the t-test (in normally distributed data with equal variances) and Mann-Whitney U-test (with unequal variances). Categorical variables were expressed in frequency and percentage and differences in frequencies were analyzed with chi-square tests. Data were expressed as mean ± standard deviation or median and ranges. P values < 0.05 were considered as stastistically significant.
3. Results
There were no statistical differences in age, gender, or body surface area between study and control groups. The age ranges of the patients with systolic impairment, without systolic impairment and control group were 9.9 ± 1.6, 7.9 ± 2.91, and 8.5 ± 3.63 years, respectively. Baseline characteristics of the study and control groups are summarized in Table 1.
| Variables | Patients with DMD (n = 62) | Controls (n = 62) | P Value |
|---|---|---|---|
| Age, y | 9.0 (7 - 12) | 8 (6.75 - 11) | 0.84 |
| Height, m | 1.28 (1.19 - 1.41) | 1.35 (1.22 - 1.48) | 0.96 |
| Heart Rate, beats/min | 72 (69 - 76) | 73 (70 - 75) | 0.46 |
| Conventional echocardiography | |||
| LVEDd, mm | 38.7 (35.6 - 43.3) | 39.1 (36.6 - 42.4) | 0.58 |
| LVSd, mm | 25.0 (13.6 - 57.2) | 24 (12.7 - 37.2) | 0.10 |
| IVSd, mm | 7 (0.64 - 0.78) | 7.3 (0.66 - 0.80) | 0.22 |
| LVPWd, mm | 5.3 (0.48 - 0.62) | 6.2 (5.2 - 6.6) | 0.03* |
| SF, % | 33 (29.9 - 37.57) | 37 (35.21 - 39.77) | < 0.001** |
| EF, % | 59 (58.75 - 68) | 69 (66.00 - 71.00) | < 0.001** |
| LV mass, g | 70.8 (58.8 - 89.41) | 64.3 (50.74 - 88.67) | 0.013* |
| LV mass index, g/m2 | 69.0 (58.7 - 84.20) | 65.8 (59.8 - 71.6) | 0.034* |
| RWT (2*LVPWd/ LVEDd) | 0.27 (0.17 - 0.55) | 0.29 (0.21 - 0.41) | 0.57 |
| Mitral E, m/s | 0.71 (0.64 - 0.88) | 0.79 (0.68 - 0.89) | 0.56 |
| Mitral A, m/s | 0.46 (0.42 - 0.52) | 0.50 (0.43 - 0.56) | 0.95 |
| E/A ratio | 1.53 (1.45 - 1.70) | 1.60 (1.40 - 1.77) | 0.66 |
| Teicholz method | |||
| LVVD, cm | 69.7 (56.54 - 80.47) | 66.1 (52.8 - 121.0) | 0.58 |
| LVVS, cm | 22.3 (4.6 - 161.3) | 20.5 (3.9 - 41.9) | 0.10 |
| SF, % | 33.6 (9.0 - 46.7) | 37.7 (30.9 - 45.2) | < 0.001** |
| Doppler | |||
| Mitral valve PHT, ms | 48.6 (39.46 - 53.82) | 56.7 (51.43 - 62.19) | < 0.001* |
| Mitral valve area, cm2 | 4.5 (4.09 - 5.58) | 3.88 (3.54 - 4.28) | < 0.001** |
| Mitral E’, cm/s | 9 (7 - 11) | 12 (10 - 14) | < 0.001** |
| Mitral A’, cm/s | 15 (14 - 17) | 17 (16 - 19) | < 0.001** |
| E/E’ ratio | 4.83 (4.12 - 5.56) | 4.32 (3.88 - 5.03) | 0.74 |
Comparison of the Variables in Patients with Duchenne Muscular Dystrophy and Controls
3.1. Echocardiographic Characteristics of Normal and DMD Subjects
The echocardiographic properties including M-mode, 2-D Doppler and TDI measurements of the study samples were assessed. Compared with the normal subjects, DMD patients had significantly higher (P < 0.05) LVmass, LV mass index and mitral valve area (P < 0.001) (Table 1). These echocardiographic measurements were compared within study groups and control groups. However DMD patients showed significantly lower EF (P < 0.001) and SF (P < 0.001) values, than the control patients. Patients with DMD had lower values of mitral valve PHT (P < 0.001). There was no significant difference between patients and control peers in terms of other measured variables.
3.2. Comparison of Subgroups of the Patients
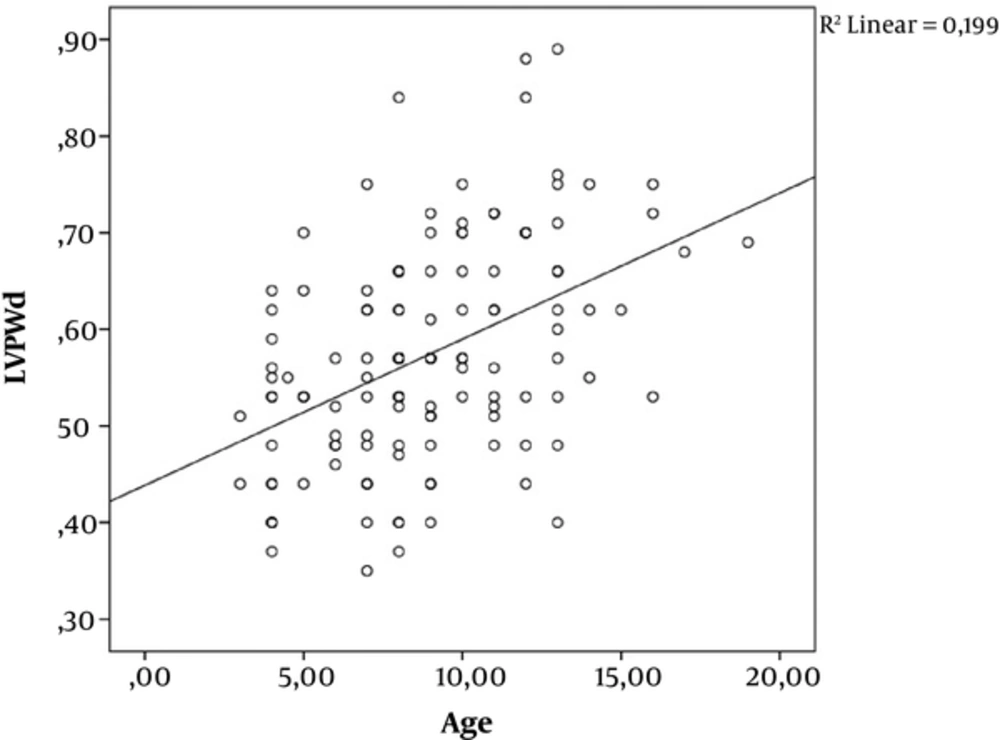
Approximately half of the study group (n=30) had normal left ventricular EF (mean ± SD; 69 ± 4.9) which constituted group Ib. The remainder of the DMD patients; group Ia had lower EF (54.9 ± 9.3). Cardiac involvement are known to become more prominent with the progression of age in DMD. Therefore group Ia patients had higher age compared to group Ib patients (P < 0.05) (Table 2). Compared with the group Ib subjects, group Ia patients had significantly higher (P < 0.01) LVH and LV mass index (Table 2). Group Ia patients also had increased LVEDd (P < 0.05), LVSd (P < 0.05), IVSd (P < 0.05) and LVPWd (P < 0.05). There was a positive correlation between age and LVPWd (P < 0,001; r = 0,446) (Figure 1).
| Variables | Group Ia EF < 60 ± 5% (n = 32) | Group Ib EF > 60 ± 5% (n = 30) | P Value |
|---|---|---|---|
| Age, y | 9.9 ± 0.16 (4 - 17) | 7.9 ± 2.9 (3 - 14) | 0.026* |
| Length, m | 1.3 ± 0.16 (1.1 - 1.6) | 1.2 ± 0.13 (1 - 1.5) | 0.05* |
| Weight, kg | 29 ± 13.9 (11 - 78) | 23 ± 8.1 (12 - 53) | 0.07 |
| Heart rate, beats/min | 72 ± 4.3 (60 - 81) | 72 ± 4.3 (60 - 81) | 0.713 |
| Conventional Echocardiography | |||
| Ejection fraction, % | 54 ± 9.3 (20 - 59) | 69 ± 4.9 (64 - 83) | 0.03* |
| Fractional shortening, % (m mode) | 29 ± 6.4 (9.4 - 32) | 37 ± 3.4 (33 - 46) | 0.002** |
| LVEDd, mm | 41.9 ± 8 (29 - 63) | 37.6 ± 4 (24 - 45) | 0.037 |
| LVSd, mm | 29.7 ± 8.6 (20 - 57) | 23.4 ± 3.4 (13 - 29) | 0.01* |
| IVSd, mm | 7.8 ± 0.1.3 (5.5 - 11) | 6.9 ± 0.9 (5 - 8.8) | 0.03* |
| LVPWd, mm | 7.6 ± 1.4 (3.5 - 8.9) | 5.4 ± 0.9 (3.7 - 7.5) | 0.04* |
| LV mass, g | 83 ± 44.2 (33 - 205) | 62 ± 19.2 (24 - 104) | 0.01* |
| LV mass index, g/m2 | 75 ± 40.2 (43 - 229) | 67 ± 19.1 (31 - 122) | 0.05 |
| RWT (2*LVPWd/ LVEDd) | 0.27 ± 0.08 (17 - 55) | 0.29 ± 0.05 (22 - 42) | 0.07 |
| Mitral valve Doppler E wave, cm/s | 8 ± 0.2 (5 - 13) | 7.2 ± 0.13 (5 - 11) | 0.06 |
| Mitral valve Doppler A, cm/s | 4.9 ± 1.0 (3.2 - 7.2) | 4.5 ± 0.7 (3.2 - 6.2) | 0.15 |
| Mitral valve Doppler E/A | 1.6 ± 0.2 (1.3 - 2.3) | 1.5 ± 0.16 (1.3 - 2.0) | 0.783 |
| Teicholz method | |||
| LVVD (Teicholz method) | 83 ± 42 (33 - 206) | 62 ± 16.7 (22 - 95) | 0.15 |
| LVVS (Teicholz method) | 39 ± 33 (13 - 161) | 19.7 ± 6.5 (4.6 - 32.7) | 0.001** |
| Fractional shortening, % | 29 ± 6.6 (9 - 42) | 37.5 ± 3.7 (30.7 - 46.7) | 0.002** |
| Doppler | |||
| Mitral valve PHT, ms | 45.6 ± 10.7 (24 - 72) | 49.2 ± 12 (31 - 78) | 0.418 |
| Mitral valve area, cm2 | 4.6 ± 1.4 (3.0 - 8.9) | 4.7 ± 1.1 (2.8 - 6.9) | 0.08 |
| Mitral E’, cm/s | 15 ± 1.3 (13 - 19) | 16 ± 3.0 (12 - 24) | 0.001** |
| Mitral A’, cm/s | 13 ± 1.5 (11 - 18) | 9.8 ± 1.9 (6 - 14) | 0.002** |
| TDI mitral valve E/ E’ | 5.10 (4.35 - 6.29) | 4.45 (3.54 - 4.22) | 0.006** |
Consequently, LV mass index was significantly increased in group Ia patients compared to other groups (P < 0.05) (Table 2). The other parameter of hypertrophy was RWT which allows to classify the hypertrophy pattern as concentric (if RWT > 0.42) or eccentric (if RWT < 0.42). In both 3 groups of this study, RWT was under the ratio of 0.42 and there was no statistically significant difference in RWT values between study and control groups. Left ventricular hypertrophy or increase in mass can change the left ventricular geometry that generally occurs as an adaptive mechanism to various physiological and pathological conditions. The LVmass was incresed in group Ia patients as mentioned before. The LV diastolic volume calculated by 2-D echocardiography was significantly increased in group I patients (P < 0.05).
3.3. Doppler and TDI Paremeters
Comparison of mitral inflow patterns ıncluding E wave, A wave and E/A ratio of the DMD patients and control peers, there was no statistically significant difference. Pressure half time measured from the mitral inflow was lower in group Ia than in other groups (P > 0.05) (Table 2). There was also significant difference between group Ib and control groups in terms of PHT (P < 0.05). We also measured mitral valve area from mitral inflow waves by Doppler technique. Mitral valve area was significantly increased in both DMD groups compared with the values obtained by controls. In addition patients with systolic impairment showed strictly increased MVA values compared with remaining DMD patients (P < 0.05).
Tissue Doppler imaging was used to measure peak myocardial velocities which showed alterations in regional myocardial function. We evaluated the diastolic velocities of mitral lateral annulus. Among the myocardial tissue velocities, patients in group Ia had lower values of indices measured with tissue Doppler, Mitral E’ (P < 0.001), Mitral A’ (P < 0.05) than those in group Ib. The ratio of E/E’ showed an increase in group Ia compared with group Ib patients.
4. Discussion
We found that DMD patients had lower EF than control patients. Also older DMD patients had impairment in systolic functions and these patients had strictly increased MVA values compared with remaining DMD patients.
Cardiac involvement develops in about 90% of DMD patients during the course of the disease (6). Although prior reports suggested that cardiomyopathy (CMP) develops with increasing age, the age at which the DMD-associated cardiomyopathy becomes clinically evident is variable (7). With the advent of cardiac fibrosis systolic dysfunction can cause disability and death between 2nd and 3rd decade of life. However some young patients may develop evident CMP before the development of exact cardiac fibrosis. We also observed systolic dysfunction in three DMD patients under 10 years. However 18 patients over the age of 10 years had no signs of systolic dysfunction in our study. MRI studies also showed that fibrotic replacement started under 10 years old but it takes a time to cause evident systolic dysfunction. Thus, a different mechanism from fibrotic replacement may cause cardiac dysfunction in young DMD patients. As suggested before, some genotypes may associate with early onset of cardiomyopathy and others may protect the early cardiac dysfunction (24, 25).
However, the recent report by Ashwath et al. (26) suggested no correlation between genotyping and cardioprotective effect. Recent genetic testing did not provide predictive information about cardiac involvement, this primarily was used to diagnose the dystrophinopathy (6). Cardiac involvement was usually determined by echocardiography. The electrocardiogram and Holter monitoring were used to determine the early signs of cardiomyopathy and cardiac autonomic dysfunction (27). In our previously published study autonomic dysfunction was detected to be developed in the earlier period of DMD before the development of mechanical cardiac dysfunction (28).
In addition, early detection of DMD-associated cardiomyopathy leads to early initiation of treatment which may slow cardiac remodeling and delay heart failure symptoms.
In the current study continuous wave Doppler signal of transmitral flow derived from mitral valve area was significantly increased in DMD patients compared with controls. In DMD subgroups, patients with systolic impairement had significantly highest MVA than remaining patients. Also this group had increased LVEDd (P < 0.05), LVSD (P < 0.05), IVSd (P < 0.05) and LVPWd. This observation can support that left ventricular dilatation similar to other causes of dilated cardiomyopathy leads to dilatation of mitral valvular annulus in DMD.
DMD-associated heart diseases are usually expressed as dilated cardiomyopathies. However some authors have reported that other types of CMP such as hypertrophic pattern or non-compaction type have been determined in patients with DMD. The best established indicator of fibrotic process in myocardium is LGE. MRI studies showed that LGE-positivity occurs early but become more prevalent with increasing age and decreasing LV ejection fraction (7).
DMD-associated CMP is characterized by fibrotic replacement of dead cells and compensatory hypertrophy of remaining cardiomyocytes. We used M-mode echocardiography to determine left ventricular chamber and wall dimensions. We also observed LV mass index was significantly higher in DMD patients. In addition, DMD patients with systolic impairment had higher LV massi values than remaining DMD patients. Increase in left ventricular mass may be related to maladaptive remodelling induced by dystrophinopathy. Loss of cardiomyocytes leads to inreased ventricular dimension that causes a rise in wall stress. Consequently LV wall hypertrophy develops to compensate increases in wall stress (29). However maladaptive remodeling occurs when increase in LV volume is disproportionate to LV wall thickness, resulting in a dilated cardiomyopathy phenotype. LV hypertrophy is an adaptive process response to variable clinical situations. Our findings supported that DMD associated CMP demonstrates a unique remodeling pattern distinct from DCM. Plausible explanation for distinct mechanism for DMD associated CMP is the fibrotic process that is not distributed uniformly in the myocardium (30). Recent reports suggest that diastolic abnormalities exist early in the setting of normal chamber size and LV systolic function (19). In the current study conventional Doppler signals measured by the mitral inflow differed significantly between study and the control groups. Doppler tissue imaging showed that diastolic functions began to deteriorate in the early phase of cardiac involvement. The hypothesis by Markham et al. (31) regarding diastolic dysfunction in the early stages of DMD is that dysfunction of the active component of diastole results in alterations in calcium homeostasis leading to impaired ventricular relaxation. Nevertheless, progression of the myocardial fibrosis contributes to impairment in the passive component of diastole leading to detoriate ventricular compliance and the development of systolic dysfunction. In this study we also found E/E’ reflecting the ventricular compliance was higher in DMD patients with lower EF. In substance cardiac involvement is at celullar level yet, when diastolic functions begin to deteriorate. Thus, TDI measurments of the mitral annulus may serve to identify early cardiac decompensation.
The prognostic value of this study seems to be superior to that provided by new echocardiography methods like global longitudinal strain in follow up and will require further longitudinal follow-up data with larger DMD patient series.