1. Background
Red blood cell transfusion is crucial in some circumstances such as acute anemic patients (1). However, transfusion-associated graft-versus-host disease (TA-GVHD) is an important and fatal complication of blood transfusion with clinical manifestations including fever, skin rash, diarrhea, nausea, vomiting, hepatitis, and pancytopenia with a morbidity of about 90%, especially in immunocompromised people (2).
The first clinical TA-GVHD was reported in 1955 and later, a report of graft-versus-host disease (GVHD) was published in bone marrow transplantation (3). In 1974, GVHD was seen in normal infants after intrauterine and exchange transfusions for Rh-incompatible-hemolytic disease; and later, karyotype analysis showed that it is related to lymphocytes (4). The number of transfused lymphocyte, which is a key factor in TA-GVHD induction, should be reduced to prevent the risks of TA-GVHD, and irradiation has been the method largely used for this purpose (2).
Fortunately, TA-GVHD can be prevented by irradiation of blood products, and blood irradiation is one of the best ways of its prevention (5). For this purpose, blood and its components can be irradiated with gamma rays by 137Cs, 60Co, or x-ray sources (6). The irradiation on RBC and platelets is not a limiting factor in clinical purpose. However, the suppression of lymphocytes, as one of the most radiosensitive cells of the body, is intensive (7). The irradiation of blood transfusion is necessary for patients who are at higher risk of TA-GVHD to reduce the number of lymphocytes in donor blood (8).
For the suppression of lymphocytes, the radiation can induce DNA aberrations in these cells (9). The magnitude of the radiation effects is associated with many factors including the characteristics of the irradiated system such as its cell type, age, and chemical content (10). Even some reports are indicating the difference in radiosensitivity of different blood groups (11). Besides these biological and chemical differences, the differences in physical properties of radiation and instruments such as dose, dose rate, and radiation type can change the effect of radiation on biological systems (10).
Irradiation of blood products can be done by the sources of gamma or x-rays. Gamma rays are monoenergetic while x-rays have an energy spectrum. All of these differences imply that we need periodical revision on the protocols of blood products irradiation. Due to problems such as radioactive source age, problems in source replacement, and protection risks, gamma sources are replacing linear accelerators (Linac). According to the problems mentioned above and the fact that the issue has not been studied in Iranian populations yet, the aim of this research was to investigate the optimum Linac dose for the lymphocyte suppression in bone marrow transplantation. All patients who were candidates for transplantation, before, during, and after the transplantation gave blood products that were the population concerned for the fatal GVHD reaction.
2. Methods
2.1. Blood Samples and X-Ray irradiation
8 ml Peripheral blood samples of the healthy right-handed people with O+ blood group aged 20 - 40 years, who had consented to participate in the study, were transferred aseptically into two 10 ml polystyrene centrifuge tubes (4 ml each) containing heparin as an anticoagulant and gently mixed. Then, 20 or 25 Gy X-ray was irradiated at the rate of 3.5 Gy/min in the internal mid-plan by compact 6MV for 30*30-field size, with 8 cm-distance of the samples to the source, and 1370 monitor unit 6MV photon (Elekta compact, Sweden). The Linac is currently in use for patient treatment; it is calibrated and has its standard. It is calibrated daily based on the center protocol. The Linac absolute dose calibration was done by standard 0.6 ml cylindrical chamber (PTW 30013, Germany) and a PC electrometer, both of which had a valid calibration certificate from Iranian SSDL. The beam profile was checked by a calibrated 2D profiler (Sun Nuclear, USA).
2.2. Isolation and Culture of PBMCs
Under sterile condition, 3 ml of PBS solution was added to the tubes. The diluted blood samples were gently layered on 3.5 ml Ficoll-Hypaque® solution (Bio-West, France) and isolated by density centrifugation at room temperature (20 minutes at 700 g). The buffy coat was collected and washed with PBS (centrifuged at 250 g for 10 min) and then, resuspended in 1 ml RPMI-1640 medium (PAA, Austria). The cell viability was determined soon after using trypan blue dye exclusion staining.
The cells were seeded in a 96-well plate (105 cells per well) and 200 μl of complete medium supplemented with/without PHA was added to each well. Then, the cells were incubated in humid air containing CO2 (5%) at 37oC.
2.3. Viability Assay Using MTT Assay
After 48 h incubation, 50 μl of MTT solution (5mg/ml in PBS) was added to each well; after 3.5 h incubation at 37˚C, 50 μl of the supernatant gently was removed, and 150 μl of acidic isopropanol (0.04 N HCl) was used to dissolve the formazan precipitate. Then, the optical density was read at 570 nm using an ELISA reader (rayto, China) and 630 nm was used as the reference wavelength.
2.4. Viability Assay Using Trypan Blue (TB) Assay
After 48 h incubation, the PBMCs were thoroughly resuspended by moving the media up and down and soon after, the viable cells were counted using TB dye exclusion technique.
The results were analyzed by SPSS version 16 using one-way ANOVA test. The research was approved by the ethics committee of our institute.
3. Results
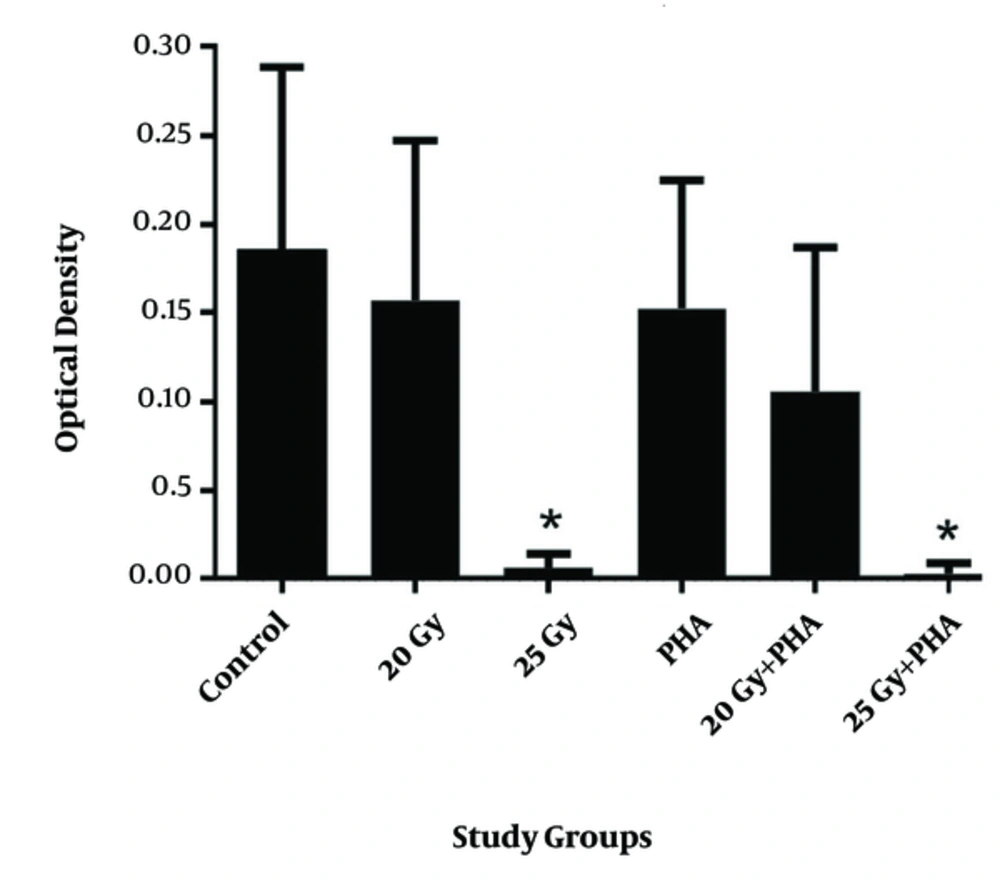
The MTT assay results for 25 Gy are depicted in Figure 1. A significant difference was observed between 25 Gy irradiated and non-irradiated samples. The results demonstrate that the dose of 25 Gy at 3.5 Gy/min in the internal mid-plan by compact 6MV for 30*30-field size, with 8 cm distance of the samples to the source, and 1370 monitor unit completely suppressed lymphocytes.
However, the difference was not significant for 20 Gy irradiation. Under the same conditions and 1096 monitor unit for total 20 Gy, we did not find optimal results.
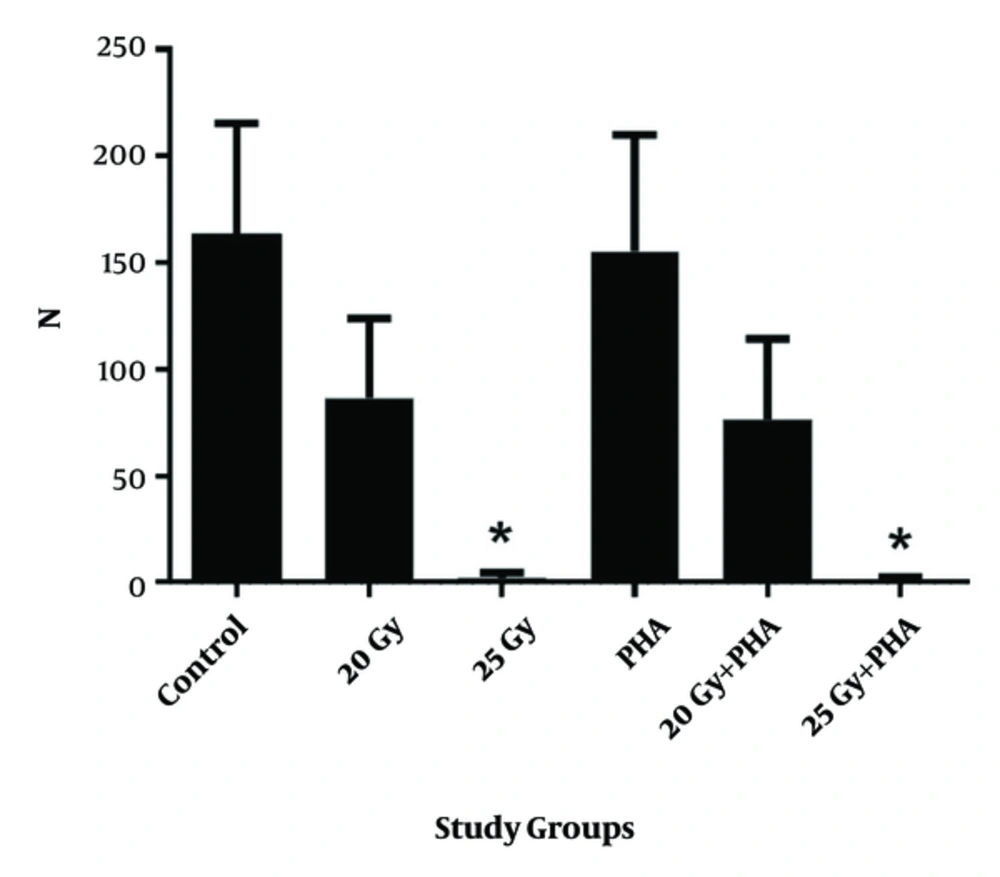
The viability of the cells obtained by the TB method (Figure 2) was completely in agreement with that obtained by MTT assay. The result of 25 Gy irradiation of blood products resulted in a significant suppression of lymphocytes. Nevertheless, 20 Gy irradiation in the above-mentioned conditions did not result in the significant suppression of lymphocytes compared to the control group. Both MTT and TB assays revealed that cytotoxicity of PHA is not significant at these doses and they did not affect the lymphocyte suppression under radiation conditions.
4. Discussion
An important point in the exposure of blood and blood products by a linear accelerator is to determine the appropriate dose of lymphocyte suppression without damage to other blood cells, and the other is designing an appropriate exposure manner. In this research, we found that the linear accelerator with specific exposure condition is a suitable alternative for the use in bone marrow transplantation and this condition of irradiation can be used. There is no similar work in this region (Iran), and because of differences in genetic and race factors, the results of this study could complete the other documents. The current study presented precisely with details that irradiation with 25 Gy at 3.5 Gy/min in the internal mid-plan by compact 6MV for 30*30 field size at 8 cm distance of the samples to the source and 1370 monitor unit completely suppress lymphocytes. The results could be useful in the middle east region and neighbors as an important step in designing an appropriate exposure manner. We selected the most resistant blood group against radiation according to previous studies (11, 12), making a difference between our study and other similar studies. The other distinction of our study was the age range that was selected in our study from 20 to 40 years.
Lymphocytes as a part of the immune system are one of the most sensitive cells to radiation. The different types of lymphocytes could serve in innate or adaptive immunity (13). These cells could be suppressed by irradiation because of their higher radiosensitivity to prevent GVHD. The lymphocytes depletion starts at low doses of radiation; but to prevent GVHD, we prefer to suppress almost all of the lymphocytes in the blood components of the donor (14).
Valerius et al. in 1981 studied the response mechanism of lymphocytes against mitogen agents after irradiation, finding that at least 20 Gy is needed for suppression (15). However, after the occurrence of GVHD, this dose was questioned (16). Another study concluded that 30 Gy gamma radiation is needed to eliminate lymphocytes (17). The Japan Society of Blood Transfusion released guidelines for the irradiation of blood, and it was updated several times. As the society recommended, we need to revise the guidelines of blood irradiation to reduce the risk of TA-GVHD (18).
Our results showed that the viability of cells decreased as a function of the radiation dose. In our study, the viability of cells after receiving 25 Gy x-ray was significantly lower than the viability at 0 and 20 Gy. The decrease in viability is in agreement with the literature. However, different conditions such as radioprotector and radiosensitizer agents (19), radio-adaptive dose and dose rate, (10), and other factors could lead to different results and the modulation in the effect of radiation could occur at higher doses.
Our study indicated that to overcome the transfusion-associated graft-versus-host disease concern, the required dose is 25 Gy at the rate of 3.5 Gy/min in the internal mid-plan by compact 6MV for 30*30 field size at 8 cm distance of debt to the tray and 1370 monitor unit. Davey reported that 2500 cGy would inactivate donor lymphocytes and prevent TA-GVHD, which is in agreement with our results (20). Góes et al. reported that at 25 Gy radiation dose, no growth was detected while at lower doses, T cells growth was observed (21). Rosen et al. concluded that a nominal dose of 2898 cGy is needed to achieve this goal (17).
Our study showed that the required radiation dose to eliminate GVHD by LINAC is 25 Gy, which is in agreement with earlier studies. We conducted the viability assay using both TB and MTT assays that gave similar results. The study could continue with different conditions such as higher x-ray energy in future.
In our study, we aimed to dysfunction leukocytes to prevent GVHD, which can occur by injecting blood, platelet, and plasma. For leukocytes and other types of infusion, we did not irradiate these products and we did not study other types of transfusions, such as leukocyte and granulocyte infusion. However, the GVHD for other types of transfusion is a concerning issue, too. We suggest studying the concerning issue of GVHD for other types of transfusions such as granulocyte and lymphocyte transfusions, especially in immunocompromised patients.
In this research, we found that a linear accelerator with specific exposure condition is a suitable alternative for Cobalt-60 sources and the above-mentioned condition of irradiation can be used to overcome GVHD concern.