1. Background
The preterm infants with birth weight less than 1500 g were defined as very low birth weight preterm infants (VLBWIs). VLBWIs may have suck-swallow un-coordination, and deeply immature gastric and intestinal functions (1). Therefore, full enteral nutrition (EN) cannot be established immediately. Parenteral nutrition (PN) is a ‘bridge’ that establishes enteral nutrient intakes and improves the VLBWIs’ growth (2). Previous studies suggested that most VLBWIs compared with the median birth weight of a reference fetus with the same postmenstrual age did not receive catch-up growth in neonatal intensive care unit (NICU) during hospitalization (3, 4). This deficiency manifested as a growth curve falling and a falling Z-score [standard deviation score (SDS)] during the early postnatal period. Z-score (SDS) is used to describe the size (weight, length, and head circumference) of an infant compared with other infants of the same age and sex (5). For example, positive SDS is greater than the 50th percentile, 0 SDS is equivalent to the 50th percentile and negative SDS is less than the 50th percentile on a growth curve. A Z-score change of zero represents unchanged or stable growth status. The Z-score was considered to be an indicator to evaluate the individual or groups’ growth. Ong et al. (6) found that 0.67 SDS represents the width of each percentile band on standard growth charts, i.e., 2nd to 9th percentile, 9th to 25th, 25th to 50th, etc. It could be a dynamic index to evaluate the growth status of preterm infants.
In today’s NICUs, especially in developing countries such as China, the problem of postnatal growth failure is still serious in VLBWIs (7). In this retrospective study, we used Δ weight SDS between birth and hospital discharge to investigate the differences in specific factors that relate to VLBWIs’ weight Z-score changes during the entire hospital stay.
2. Methods
2.1. Study Population
Following approval of the institutional review board, the medical record was searched to identify patients evaluated at the First Affiliated Hospital of Sun Yat-sen University with a diagnosis code. This retrospective study was performed at a single university-based NICU (the First Affiliated Hospital of Sun Yat-sen University). The nutritional protocol and recordkeeping were provided as standard care in our department. The university hospital perinatal database was queried to identify and obtain the demographics of VLBWIs (birth weight < 1500 g, gestational age < 37 weeks, and hospitalized within 24 h of birth) admitted from 1 June 2010 to 30 March 2017. Infants who had cardiac anomalies, congenital gastrointestinal anomalies, or hereditary metabolic diseases; were transferred after 24 postnatal hours, died in the hospital, had a hospital stay of < 15 days, or had unstable vital signs upon hospital discharge were excluded.
Infant demographics, including Z-score (SDS) for weight, length, and head circumference between birth and discharge using the Fenton 2013 growth chart (8), as well as age upon initiation, were obtained. All infants were divided into two groups (non-catch-down [NCD] group: Δ weight SDS ≥ -0.67; catch-down [CD] group: Δ weight SDS < -0.67). All data were compared between the two groups for further evaluation.
2.2. Perinatal Characteristics and Complications
The data of maternal characteristics and complications that affected the wellbeing of an infant, such as gemellary pregnancy, pregnancy-induced hypertension, steroid use, delivery route, premature rupture of membrane, fetal distress, in vitro fertilization and embryo transfer, intracytoplasmic sperm injection, twin-twin transfusion syndrome and multifetal reduction were analyzed. Additionally, the data of infants’ characteristics and complications such as small for gestational age (SGA), bronchopulmonary dysplasia (BPD), sepsis, necrotizing enterocolitis (NEC), intraventricular hemorrhage (ICH), retinopathy of prematurity (ROP), extrauterine growth restriction (EUGR), and parenteral nutrition associated cholestasis (PNAC) for the two groups were also recorded. Total days of return to birth weight and hospital stay were calculated and analyzed.
2.3. Anthropometric Parameters at Birth and Discharge
The infants’ weight, length, and head circumference and Z-score (SDS) for weight, length, and head circumference at birth and discharge were also obtained and studied.
2.4. Nutrition Management
Daily intakes of all nutrients including parenteral nutrition (PN) and enteral nutrition (EN) recorded in patient charts according to NICU protocol were assessed. After birth, PN was initiated within 24 hours and contained glucose, amino acids, and lipids in our NICU. Energy was increased daily based on metabolic tolerance. The protein content and energy density of enteral nutritional intake were calculated according to the guideline (2). Formula milk composition was calculated by using the manufacturers’ datasheets. Total days of PN and laboratory biochemical indicators (blood urea nitrogen [BUN], creatinine [Cr], aspartate transaminase [AST], and alanine transaminase [ALT]) during hospitalization were also obtained.
2.5. Statistical Analyses
Baseline characteristics, daily changes in protein and energy intake with PN and EN, clinical outcomes, and complications during the NICU stay were compared between the two groups. Continuous variables are presented as mean ± standard deviation or median or quartile range (P25, P75). Student’s t-test was used to compare quantitative data with a normal distribution. Parameters without a normal distribution were analyzed by the Mann-Whitney U test. Categorical variables such as maternal and clinical outcomes of the two groups are presented as a percentage that was analyzed by chi-square test and Fisher’s exact test. Calculations were performed using SPSS software version 17.0 (SPSS Inc, Chicago, IL, USA). The level of significance was set at a P < 0.05.
3. Results
3.1. Perinatal Characteristics and Complications
A total of 227 VLBWIs (NCD group = 117, CD group = 110) met the inclusion criteria. Perinatal characteristics and complications are listed in Table 1. No significant differences in maternal characteristics and complications were found between the two groups. The incidence of most common complications (except for BPD and PNAC) related to prematurity and mortality was not significantly different between the two groups. The incidence of BPD (0.348 vs 0.518, respectively; P < 0.05) and PNAC (0.196 vs 0.341, respectively; P < 0.05) were significantly lower in the NCD group than in the CD group. The mean PN duration, length of hospital stay, and mean days of return to BW were significantly shorter in the NCD group (P < 0.05).
| NCD Group (n = 117) | CD Group (n = 110) | t/z/χ2 | P Values | |
|---|---|---|---|---|
| Maternal | ||||
| Gemellary pregnancy | 58 (49.57) | 60 (54.55) | 0.562 | 0.454 |
| Pregnancy-induced hypertension | 29 (24.79) | 30 (27.27) | 0.182 | 0.669 |
| Prenatal steroids | 62 (52.99) | 49 (44.55) | 1.619 | 0.203 |
| Cesarean delivery | 21 (17.95) | 23 (20.09) | 0.318 | 0.573 |
| PROM | 41 (35.04) | 35 (31.82) | 0.265 | 0.607 |
| Fetal distress | 18 (15.38) | 20 (18.18) | 0.318 | 0.573 |
| Meconium stained amnionic fluid | 12 (10.26) | 14 (12.73) | 0.341 | 0.559 |
| Placenta praevia | 11 (9.40) | 10 (9.09) | 0.007 | 0.936 |
| IVF-ET | 25 (21.37) | 26 (23.64) | 0.168 | 0.682 |
| ICSI | 4 (3.42) | 3 (2.73) | 0.091 | 0.763 |
| TTTS | 10 (8.55) | 15 (13.64) | 1.498 | 0.221 |
| Multifetal reduction | 9 (7.69) | 10 (9.09) | 0.145 | 0.704 |
| Infant | ||||
| GA, w | 30.2 (29.0, 32.5) | 30.4 (28.6, 32.3) | 1.109 | 0.282 |
| SGA | 42 (35.90) | 44 (40.00) | 0.406 | 0.524 |
| RDS | 104 (88.89) | 100 (90.91) | 0.254 | 0.614 |
| Apnea | 27 (23.08) | 29 (26.36) | 0.330 | 0.566 |
| Pulmonary infection | 45 (38.46) | 56 (50.91) | 3.557 | 0.059 |
| BPD | 49 (41.88) | 65 (59.09) | 6.717 | 0.010 |
| NEC | 23 (19.65) | 22 (20.00) | 0.004 | 0.949 |
| Sepsis | 36 (30.77) | 31 (28.18) | 0.344 | 0.558 |
| ICH | 36(30.77) | 29 (26.36) | 0.539 | 0.463 |
| ROP | 38 (32.48) | 34 (30.91) | 0.064 | 0.800 |
| PNAC | 39 (33.33) | 55 (50.00) | 0.210 | 0.646 |
| EUGR | 28 (28.3) | 54 (60.0) | 15.553 | 0.000 |
| Duration of PN, d | 31 (24,41) | 40 (29,52) | 3.487 | 0.000 |
| Return to BW, d | 6 (1,8) | 8 (1,11) | 2.256 | 0.028 |
| Hospital stay, d | 39 (30,52) | 49 (38,62) | 3.259 | 0.001 |
Main Perinatal Complications and Complicationsa
3.2. Anthropometric Parameters at Birth and Discharge
The groups were compared for anthropometric parameters and Z-score (SDS) at birth and discharge (Table 2). No significant differences were found between the two groups at birth. The anthropometric parameters at discharge were significantly higher in the NCD group (P < 0.05). Additionally, the SDS for weight and length and head circumference at discharge was significantly higher in the NCD group (P < 0.05).
| NCD Group (n = 117) | CD Group (n = 110) | t/z | P Value | |
|---|---|---|---|---|
| Weight at birth, kg | 1.22 ± 0.23 | 1.19 ± 0.21 | 1.024 | 0.153 |
| Weight SDS at birth | -0.64 ± 0.51 | -0.56 ± 0.45 | 1.255 | 0.105 |
| Length at birth, cm | 27.6 ± 1.6 | 27.5 ± 1.7 | 0.457 | 0.324 |
| Length SDS at birth | -0.64 ± 0.37 | -0.62 ± 0.34 | 0.423 | 0.336 |
| HC at birth, cm | 37.6 ± 3.4 | 37.9 ± 3.5 | 0.655 | 0.257 |
| HC SDS at birth | -0.37 ± 0.29 | -0.34 ± 0.25 | 0.832 | 0.203 |
| Weight at discharge, kg | 2.48 ± 0.41 | 2.22 ± 0.81 | 3.022 | 0.003 |
| Weight SDS at discharge | -0.75 ± 0.35 | -0.97 ± 0.87 | 2.471 | 0.015 |
| Length at discharge, cm | 45.8 ± 2.8 | 43.4 ± 3.6 | 4.256 | 0.000 |
| Length SDS at discharge | -0.41 ± 0.65 | -1.29 ± 0.48 | 4.707 | 0.000 |
| HC at discharge, cm | 31.4 ± 2.5 | 30.4 ± 1.7 | 3.542 | 0.000 |
| HC SDS at discharge | -0.81 ± 0.76 | -1.12 ± 0.64 | 3.314 | 0.001 |
Anthropometric Parameters at Birth and Dischargea
3.3. PN and EN Intake
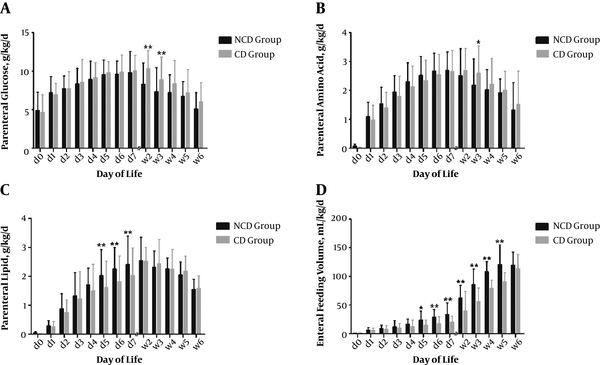
Daily changes in protein and energy intake from birth to the sixth week of life are presented in Figures 1 and 2. During the first week of life, the daily average parenteral amino acid (g/kg/d) and glucose (g/kg/d) administered to the two groups were not significantly different, while parenteral lipids (g/kg/d) administered to the NCD group were significantly higher than those of the CD group from day 5 to day 7 (P < 0.05) (Figure 1A - 1C). After the first week of life, the NCD group received significantly lower parenteral glucose (g/kg/d) from week 2 to week 3 and lower parenteral amino acid (g/kg/d) at week 3 (P < 0.05) (Figure 1A and 1B).The NCD group received higher mean daily enteral feeding volume (mL/kg/d) than the CD group from day 5 to week 5 (P < 0.05) (Figure 1D).
Mean daily PN, EN intakes and protein energy ratio from birth to 6th week from birth to the sixth week. During the first week of life, the daily average parenteral amino acid (g/kg/d) and glucose (g/kg/d) administered to the 2 groups were not significantly different, while parenteral lipids (g/kg/d) administered to the NCD group were significantly higher than those of the CD group from day 5 to day 7 (A - C). From week 2 to week 3, the NCD group was administered significantly lower parenteral glucose than the CD group (A). At week 3, the NCD group was administered significantly lower parenteral amino acid (g/kg/d) than the CD group (B). The NCD group was administered a higher mean daily enteral feeding volume (mL/kg/d) than the CD group from day 5 to week 5 (D). Error bars represent standard deviations; *P < 0.05; **P < 0.01.
Total protein intake and total energy intake via PN and EN from birth to the sixth week. A, the NCD group had a significantly increased total protein intake (g/kg/d) during day 5 to day 6 and week 4 to week 5. The NCD group had a greater mean daily enteral protein intake from day 5 to week 5. The CD group had increased parenteral protein intake week 2 to week 5 and significantly higher than the NCD group during week 3; B, the NCD group had a significantly increased energy intake (kcal/kg/d) during the first 6 weeks of life, except for the first four days. The NCD group had higher parenteral energy than those of the CD group from day 5 to day 7. The CD group had increased parenteral energy intake week 2 to week 5 and significantly higher than the NCD group from week 2 to week 4. The P:E ratio was higher at the first week and significantly higher at day 2 to day 4 and day 7 in the NCD group (2C). During week 2 to week 3, the P:E ratio was significantly lower in the NCD group (C). Error bars represent standard deviations; *P < 0.05, **P < 0.01 for EN; ΔP < 0.05 for PN; #P < 0.05 for total protein /energy intake; &P < 0.05, &&P < 0.01 for the P:E ratio.
The NCD group had a greater mean daily EN protein intake (mL/kg/d) from day 5 to week 5 (P < 0.05) (Figure 2A). The NCD group had higher parenteral energy (kcal/kg/d) than that of the CD group from day 5 to day 7 (P < 0.05) (Figure 2B). Moreover, the NCD group had a significantly increased energy intake (kcal/kg/d) during the first six weeks of life (P < 0.05), except for the first four days (Figure 2B). The P:E ratio was higher at the first week and significantly higher at day 2 to day 4 and day 7 in NCD group (P < 0.05) (Figure 2C). However, from week 2 to week 3, the P:E ratio was significantly higher in the CD group (P < 0.05) (Figure 2C).
3.4. Main Laboratory Test
No differences were found between the two groups regarding mean values of the main laboratory tests from the first to the sixth week of life (Table 3).
| NCD Group (n = 117) | CD Group (n = 110) | t | P Value | |
|---|---|---|---|---|
| Week 1 | ||||
| BUN, mmol/L | 4.14 ± 1.45 | 4.09 ± 1.60 | 2.247 | 0.805 |
| Cr, umol/L | 73.23 ± 8.22 | 74.66 ± 8.75 | 1.267 | 0.103 |
| AST, U/L | 30.86 ± 6.45 | 30.32 ± 7.10 | 0.599 | 0.275 |
| ALT, U/L | 13.42 ± 2.18 | 13.48 ± 1.91 | 0.221 | 0.413 |
| Week 2 | (n = 117) | (n = 110) | ||
| BUN, mmol/L | 4.23 ± 2.04 | 4.12 ± 1.94 | 0.416 | 0.678 |
| Cr, umol/L | 55.47 ± 5.12 | 54.69 ± 5.67 | 1.086 | 0.139 |
| AST, U/L | 27.37 ± 3.28 | 26.92 ± 7.63 | 0.571 | 0.284 |
| ALT, U/L | 8.59 ± 3.64 | 8.33 ± 3.81 | 0.525 | 0.300 |
| Week 3 | (n = 106) | (n = 102) | ||
| BUN, mmol/L | 3.24 ± 1.17 | 3.32 ± 1.02 | 0.531 | 0.596 |
| Cr, umol/L | 34.12 ± 4.34 | 35.46 ± 4.68 | 0.543 | 0.294 |
| AST, U/L | 25.10 ± 10.52 | 26.44 ± 9.73 | 0.954 | 0.171 |
| ALT, U/L | 9.05 ± 4.67 | 9.58 ± 5.56 | 0.752 | 0.453 |
| Week 4 | (n = 69) | (n = 75) | ||
| BUN, mmol/L | 3.04 ± 1.28 | 3.26 ± 1.01 | 1.138 | 0.257 |
| Cr, umol/L | 36.53 ± 6.21 | 37.45 ± 6.70 | 0.855 | 0.197 |
| AST, U/L | 25.24 ± 5.53 | 25.59 ± 5.56 | 0.378 | 0.353 |
| ALT, U/L | 9.12 ± 3.48 | 9.14 ± 4.25 | 0.031 | 0.488 |
| Week 5 | (n = 40) | (n = 53) | ||
| BUN, mmol/L | 3.68 ± 1.59 | 3.54 ± 1.39 | 0.452 | 0.652 |
| Cr, umol/L | 31.32 ± 5.95 | 31.78 ± 6.01 | 0.368 | 0.357 |
| AST, U/L | 24.74 ± 3.24 | 25.50 ± 3.15 | 1.133 | 0.131 |
| ALT, U/L | 16.39 ± 4.68 | 16.68 ± 3.85 | 0.319 | 0.375 |
| Week 6 | (n = 30) | (n = 39) | ||
| BUN, mmol/L | 3.20 ± 1.96 | 3.33 ± 1.55 | 0.3.8 | 0.759 |
| Cr, umol/L | 31.58 ± 3.29 | 30.71 ± 4.77 | 0.895 | 0.187 |
| AST, U/L | 27.24 ± 3.17 | 27.58 ± 3.63 | 0.415 | 0.340 |
| ALT, U/L | 18.69 ± 6.84 | 19.74 ± 6.40 | 0.650 | 0.259 |
Mean Laboratory Values from the First Week to the Sixth Week of Lifea
4. Discussion
Our study demonstrated that the NCD group was given more parenteral lipid and total energy at the first week, and received earlier enhanced enteral feeding volume than the CD group during hospital stay. This study revealed that parenteral and enteral nutrition might play the most important role in the postnatal growth of VLBWIs during hospitalization. The improved weight Z-score of VLBWIs during hospital stay might associate with the earlier adequate parenteral lipid and energy intake at the first week of life and enhanced enteral feeding volume during hospital stay. The infants with significant decline in weight Z-scores might be more prone to developing BPD and PNAC.
Parenteral lipids are one of the most important energy providers (9), that can provide calories required for the process of protein synthesis. Increased parenteral lipids significantly improve nitrogen utilization and retention, resulting in positive nitrogen balance in preterm VLBWIs (10). In our study, we found that parenteral lipids and total parenteral energy administered to the NCD group were significantly higher than those administered to the CD group from day 5 to week 1. Similar to our findings, researches on parenteral lipids had shown that higher dosages of intravenous lipids helped to decrease postnatal weight loss and have an earlier birth weight regain (11, 12). Dit Trolli et al. (13) demonstrated that early lipid supply (lipids were initiated at the first three days after birth and increased 0.5 to 1 g/kg/d to reach 4 g/kg/d) during the first two weeks of life could improve the weight gain from birth to day 28 of life and ameliorate the neurological development at a corrected age of one year for preterm VLBWIs. Moreover, recent randomized controlled trials (RCTs) have indicated that increasing parenteral energy intakes during the first few days of life significantly improve the preterm infants’ head growth (14, 15). Thus, our results add to the evidence that earlier administration of adequate parenteral lipids and energy may help to meet the numerous nutritional needs of VLBWIs.
In our study, the mean daily enteral feeding volume administered to the NCD group was higher than the CD group from day 5 to week 5. This finding indicated that an earlier and enhanced enteral feeding plays an important role in the growth for VLBWIs. Early EN is known to decrease gut atrophy and intestinal permeability (16) and has been associated with improved postnatal growth (17). A recent RCT (18) demonstrated a significantly better weight gain during hospitalization and adequate growth of head circumference in the enhanced feeding group. Enhanced enteral feeding might also improve weight gain during hospitalization (19). Morgan et al. (20) suggested that advancing enteral feed volumes (30 to 40 mL/kg/d) compared to slow enteral feed volumes (15 to 24 mL/kg) does not increase the incidence of NEC or death in VLBWIs. Additionally, they found that the slow enteral feed volumes might delay in establishing full enteral feeds and increase the risk of infection in VLBWIs. Guidelines for feeding VLBWIs of McMaster University (21) suggested that infants weighing < 1000 g at birth reach full enteral feeding (~150 to 180 mL/kg/d) by about two weeks in and by about one week in infants weighing 1000 to 1500 g by using evidence-based feeding protocols. Furthermore, we found that the NCD group received lower parenteral protein and energy intake after two weeks. We inferred that the NCD group had received higher EN during the first week, then gradually reduced PN and transited to full enteral feeding. Meanwhile, this study also suggested that enhanced enteral feeding volume during the first week might help to shorten the time of PN duration and achieve full enteral feeding quickly.
We noticed that the NCD group was administered a higher total protein intake from day 5 to week 5 after birth and had a higher P:E ratio during the first week of life. Protein is most important nutrient for the growth of preterm VLBWIs as the main functional and structural component of the body cells and structure (2). It has been estimated that early high protein intake could improve weight gain and the other growth outcomes at discharge (22). The European society for paediatric gastroenterology hepatology and nutrition (ESPGHAN) recommended that the infants up to 1000 g should receive 4.0 to 4.5 g/kg/day protein, and the infants from 1000 to 1800 g should receive 3.5 to 4.0 g/kg/day protein that will meet the needs of most preterm infants (23). The types of EN such as human milk and formula milk can meet the VLBWIs’ protein and energy needs (2). Moreover, early and reasonable energy intake could improve the preterm infants’ growth and development (24). Stephens et al. (25) found that increasing first-week protein and energy intake resulted in higher mental developmental index scores of extremely low birth weight infants (ELBWIs) at 18 months. Additionally, we found that the NCD group received a lower P:E ratio at 2 to 3 weeks. We speculated that the reduction of P:E ratio was due to the circumstance that NCD group had received higher total protein intake with higher P:E ratio at the first week, then it gradually reduced with lower proportion of protein in PN.
In this our study, the NCD group had lower incidence of BPD suggesting that BPD was closely related to greater nutritional intake. The infant with BPD has higher oxygen consumption and energy expenditure (26). Poor nutrient conditions might damage the process of immature lung tissue remodeling and develop severe chronic lung disease (26). Uberos et al. (27) suggested that lower enteral intake of energy and total lipids during the first 14 days of life increase the risk of BPD in ELBWIs. Gianni et al. (28) showed that energy intake and severity of BPD were the major factors influencing the weight gain of preterm VLBWIs. Additionally, several studies have demonstrated that the infants who have a slower weight gain may be more prone to developing infections and BPD (29, 30).
In the meantime, we found that NCD group had a lower incidence of PNAC. Prematurity, low birth weight and long PN duration in VLBWIs are often described as risk factors for developing PNAC (31, 32). In VLBWIs, the rate of PNAC has been reported to be as high as 50% (33). Longer PN duration is an independent risk factor for developing PNAC (34). Repa et al. (35) found that “aggressive” nutrition (amino acids and lipids concentrations of 2.0 g/kg/d and 1.0 g/kg/d initiated at the first day of life and increased stepwise by 0.5 g/kg/d to reach 4 g/kg/d; the increasing rate of EN was up to 20 mL/kg/d) was associated with a significant lower incidence of PNAC and improved postnatal growth of ELBWIs significantly. Previous studies provided adequate nutritional support through a combination of early PN and EN, followed by a progressive PN reduction, as enteral feeding volumes were steadily advanced to full EN (36, 37). Hamilton et al. (38) found that initiating enteral feeding within 24 hours of birth was associated with a significantly lower number of PN days. Our study suggested that in VLBWIs early administration of an enhanced EN nutritional regimen might reduce the incidence of intrahepatic cholestasis because of a shorter PN duration during hospitalization.
This study may help to add new idea to the management of VLBWIs in NICU. The VLBWIs’ adequate growth during hospitalization might associate with adequate parenteral lipids, energy, and enhanced enteral feeding volume. However, the benefits of this nutritional regimen regarding long-term, especially neurodevelopmental and metabolic outcomes, remain to be proven in adequately-powered RCT. Practicing early PN and higher EN nutrient supplementation requires further study especially in developing countries.
4.1. Conclusions
In conclusion, this study shows that the VLBWIs’ Z-score changes might significantly associate with parenteral and enteral nutrition during hospitalization. The VLBWIs’ weight Z-score might be significantly improved by the earlier adequate parenteral lipid, energy intake, and earlier enhanced enteral feeding volume. Additionally, the infants with significant decline in weight Z-scores might be more prone to developing BPD and PNAC.