1. Background
Resistin, visfatin, leptin, and adiponectin are bioactive molecules synthesized in the adipose tissue. They are referred to as adipocytokines with various actions participating in regulation of metabolic processes in the human body. Human resistin is a cystein-rich peptide produced by monocytes, macrophages, and the adipose tissue and it is also known as “Found in Inflammatory Zone” (FIZZ) (1, 2). In humans, white adipose tissue (WAT) produces only small amounts of this protein, while high levels of resistin mRNA are detectable in circulating mononuclear cells. Resistin has been reported in recent clinical trials as a predominantly pro-inflammatory protein associated with acute and chronic inflammation. It is thought to have an important role in inflammation because of its strong expression by monocytes and macrophages (3, 4).
Recent studies have suggested that some adipocytokines like resistin might play a role either in immune diseases such as rheumatoid arthritis or in cardiovascular diseases as a result of its effects in the development of endothelial dysfunction, thrombosis, angiogenesis, inflammation, and smooth muscle cell dysfunction (5, 6).
Henoch Schönlein Purpura (HSP) is one of the most common vasculitic diseases of childhood and characterized by leukocytoclastic vasculitis of capillaries. Although HSP is believed to be an Ig-A related immune-complex mediated disease, its pathogenesis still remains unclear (7). Despite the pathophysiologic importance of resistin in systemic inflammation, to our knowledge, no study so far has investigated serum resistin levels in HSP.
Taking into consideration that small-vessel IgA deposition is the hallmark of IgA vasculitis and the fact that resistin is regarded as a marker of inflammation, it would be more appropriate to state that the rationale of this "cross-sectional study" was to investigate serum and urinary resistin levels in the acute phase of childhood-diagnosed IgA vasculitis.
2. Materials and Methods
This cross-sectional study was performed between March 2015 and September 2015 at University of Health Sciences, Umraniye Training and Research Hospital. Fifteen children with HSP were evaluated during the acute phase and compared with fifteen healthy controls. The parents of all patients and control subjects were informed about the study objectives before inclusion in the study. The study was approved by the local ethics committee.
European League Against Rheumatism (EULAR) criteria were used to establish the diagnosis of HSP (8). The acute phase was defined as the time period between the onset and resolution of symptoms including rash, abdominal pain, arthritis, etc. A history of drug usage, arthralgia, seizures, insect bites, trauma, and abdominal pain were excluded. Diabetes mellitus, obesity, metabolic syndrome, heart and renal diseases were elicited from each patient. All patients underwent testing for complete blood count (CBC), serum electrolytes, blood urea nitrogen (BUN), creatinine, total protein, albumin, urinalysis, C-reactive protein (CRP), latent blood test in stool, prothrombin time, and partial thromboplastin time. All controls had a normal physical examination, normal blood pressure, serum electrolytes, BUN and creatinine levels throughout the study. We correlated acute phase reactants such as WBC, CRP as well as s itt.
Blood and urinary samples were collected from the patients with acute HSP and healthy controls at admission. Resistin levels were measured using enzyme-linked immunosorbent assay (ELISA). The samples were collected into a serum separator tube. After clot formation, they were centrifuged at 2000 × g for ten minutes. After removing serum, sera were stored at -20 °C or below. Resistin ELISA kits were purchased from ASSAYPRO LLC, Inc. (USA). This assay employs a quantitative sandwich enzyme immunoassay technique, which measures resistin level in less than 5 hours.
2.1. Statistical Analysis
In this study, the results were analyzed using IBM SPSS Statics 22 (IBM SPSS, Turkey) software package. Kolmogorov-Smirnov confirmed the normality of distribution of the study parameters.The Student’s t test was used to compare resistin levels between HSP patients and healthy controls. The Pearson correlation analysis was used to investigate the correlations between parameters. Statistical significance was determined at P < 0.05.
2.2. Results
HSP group consisted of 15 HSP patients. The mean age of the HSP group was 8.65 ± 4.35 (range 3 - 15) years, 9 of them were male (60%) and 6 (40%) were female. Nine of the 15 patients in the control group were male (60%), 6 (40%) were female; their mean age was 9.4 ± 5.6 (range 3 - 16) years.
The HSP and control groups were not signifcaintly different in terms of gender and age (P > 0.05).
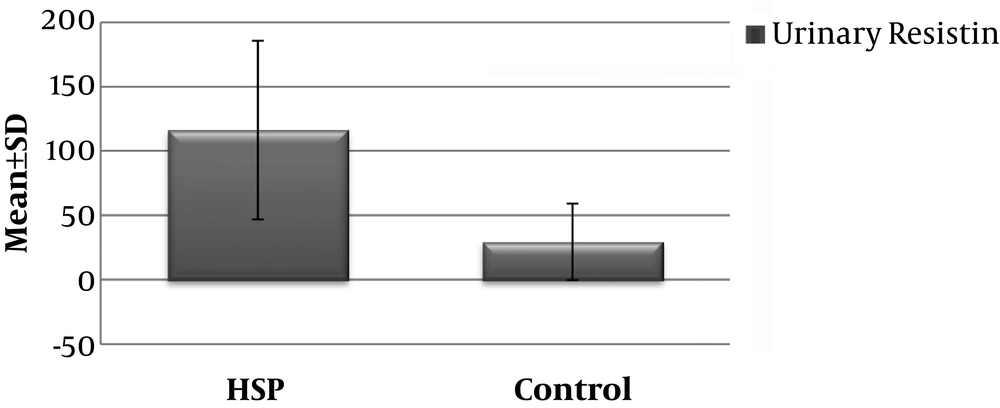
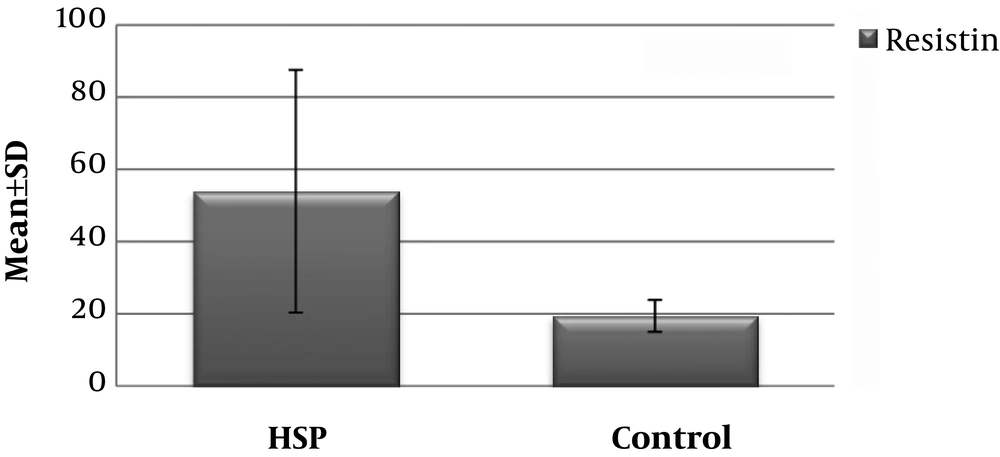
The serum resistin level of the HSP group (53.96 ± 33.61 ng/ml) was significantly higher than that of the control group (19.46 ± 4.42 ng/ml) (P < 0.001) (Figure 1) Similarly, the mean urinary resistin level of patients in the HSP group (116,36 ± 69,54 ng/ml) was significantly higher than the control group (29,39 ± 29,66 ng/ml) (P < 0.001) (Figure 2 and Table 1).
| Serum Resistin, Mean ± SD | Urinary Resistin, Mean ± SD | P | |
|---|---|---|---|
| Patients (n=15) | 53.96 ± 33.61 | 116.36 ± 69.54 | 0.001a |
| Controls (n=15) | 19.46 ± 4,42 | 29.39 ± 29.66 |
aStudent’s t-test, P < 0.01
Nine (60%) patients in the HSP group had occult blood in the stool, while 6 (40%) patients in the same group did not have any bleeding. In the HSP group there was no correlation between mean serum resistin levels and CRP, WBC (r = 0.038, P = 0.892 and r = 0.236, P = 0.343, respectively).
In the HSP group 11 (73%) patients had arthritis whereas 4 (27%) patients had it not.
Mean resistin levels were 61.47 ± 44.86 ng/ml in the arthritic subgroup and 47.39 ± 20.69 ng/ml in the non-arthritic subgroup. There was also statistically no significant difference between cases with arthritis and those without (P = 0.439) (Table 2).
| N (%) | Resistin (Mean ± SD) | P |
|---|---|---|
| Fecal occult blood test (+) 9 (60) | 48.15 ± 15.15 | 0.653 |
| Fecal occult blood test (-) 6 (40) | 56.87 ± 40.33 | |
| Arthritis (+) 4 (27) | 61.47 ± 44.86 | 0.439 |
| Arthritis (-) 11(73) | 47.39 ± 20.69 |
aStudent t-test
In the HSP group there was no correlation between mean serum resistin level and CRP or WBC (r = 0.038, P = 0.892 and r = 0.236, P = 0.343, respectively). Similarly, there was no significant correlation between serum and urinary resistin levels (r = 0.086, P = 0.761, respectively) (Table 3).
| Patients | Serum Resistin | |
|---|---|---|
| r | P | |
| CRP | 0.038 | 0.892 |
| WBC | 0.236 | 0.343 |
| Urinary Resistin | 0.086 | 0.761 |
Abbreviation: CRP, C-reactive protein
aPearson’s Correlation test
3. Discussion
In this study, we demonstrated increased serum and urinary resistin levels in patients with acute-stage HSP compared to healthy controls. To date, the effects of resistin in vasculitic disorders have not been completely understood. No study has yet investigated resistin level in HSP.
In many studies, high plasma resistin levels have been linked to impaired renal function in patients with chronic renal disease, severity of inflammation in patients with inflammatory bowel disease, and increased cardiac risk in patients with congestive heart failure (9-11).
A study dated 2010 demonstrated that 44 children with Kawasaki disease (KD) had higher serum resistin levels than the control group that regressed to normal levels after intravascular immunoglobulin treatment (12). Kemmotsu et al. (13) also found that serum resistin levels were higher in 31 patients with KD although there was no change in levels of other adipocytokines such as leptin and visfatin. The authors also reported a positive correlation between serum resistin levels and CRP. In our study, serum resistin levels of patients with HSP were significantly higher but they showed no significant correlation with CRP and WBC. We suggest that the absence of such significant correlation between acute phase reactants and serum resistin stems from the different pathophysiological mechanisms underlying vasculitis in KD and HSP. For example, significant cutaneous vascular IgA deposits are common in Henoch-Schönlein purpura but not in other vasculitides.
Schaeffler et al. (14) reported that the synovial resistin level is 10-fold higher in patients with rheumatoid arthritis than in patients with osteoarthritis. The hypothesis that resistin has proinflammatory function is also supported by the development of arthritis after resistin injection into healthy joints of mice (15).
In a study on SLE patients, serum resistin levels were correlated with inflammation severity, bone mass density, and renal function. Serum resistin level was correlated with high IgG and elevated proinflammatory cytokine levels (IL-1β, IL-6, TNFα, and soluble IL-6 receptor). It has been similarly suggested that the relationship of resistin with ESR and complement component 3 may reflect disease activity (16).
Resistin is also involved in the inflammatory process in Behçet disease where a correlation between resistin levels and disease activity has been shown (17).
Resistin has also been shown to have effects on vascular endothelial cells through increasing VCAM-1 and ICAM-1 expression and promoting endothelin-1 release, as well as its effects on inflammatory cytokines (18).
In an in vitro animal study by Cho et al. (19) resistin induced accumulation of macrophages in the intimal and adventitial layers of the carotid arterywas found as well as an increase in cytokines such as IL-1, IL-6, and TNF, and also VCAM-1 adhesion molecule.
In a large cohort study conducted by Prugger et al. (20) resistin had a more significant predictive power than traditional risk factors for ischemic stroke.
A study on adult patients with postoperative sepsis/septic shock, which was conducted in 2016, demonstrated that median plasma adinopectin levels were reduced and resistin and leptin levels were elevated in sepsis compared to preseptic plasma levels. The survivors were found to have higher levels of preseptic adiponectin than the survivors, so it was hypothesized that changes in adiponectin levels in patients with sepsis/septic shock following surgery could be used as prognostic markers (21).
In our study, serum and urinary resistin levels were significantly higher in acute stage of HSP than the in healthy control group.
A low number of subjects is a limitation of this study. However, this was a pilot study and so the relatively small number of subjects seems to be acceptable.
4. Conclusions
The effect of resistin in vasculitic disorders has yet to be elucidated. In our study serum and urinary resistin levels were significantly higher in patients with acute-stage HSP than in healthy control group. According to our results, serum and urinary resistin levels could be of use as a diagnostic or prognostic marker and be considered as a secondary epiphenomenon in children with HSP.
Further studies are warranted to elucidate the pathogenic mechanisms by which serum and urinary resistin levels elevate in HSP patients with kidney involvement and relapses.