1. Background
Pathological lesions are highly concerning in children since they affect their growth and development of the patients and may have serious consequences (1).
Head and neck region has more anatomical variations than other parts of the human body. Such variations in anatomical structures are responsible for relatively high frequency of pathological masses in the head and neck region (2). These lesions are highly variable in children as in adults (3). Head and neck masses are common in infants, children and adolescents (4, 5). Several studies have classified head and neck masses into three groups of inflammatory/reactive/infectious, congenital/developmental and neoplastic lesions; the neoplastic group is divided into two subgroups of benign and malignant lesions (4-6).
About 80 - 90% of head and neck masses in children are benign with congenital or inflammatory origin (1, 5, 7). However, many benign lesions may also have serious consequences and may even cause death if develop in critical structures (3).
According to the statistics reported by the United Nations in 2015 and 2016, the population of individuals below the age of 15 years is 26% accounting for about one-fourth of the world’s population (8). In Iran, in 2016, the population of children younger than 14 years was 24% accounting for about one-fourth of the country’s population; the population of two to 12-year-olds was 16% of the total population (9).
Comprehensive knowledge of dentists and other personnel in the medical field is important in early detection of head and neck masses and their management (4, 6, 10). Some previous studies on cervical masses in children are available but studies on the head and neck masses are limited (4, 10, 11). Considering the reported statistics and role of race, ethnicity and geographical location in the prevalence of these lesions (6, 11), this study aimed to determine the frequency of head and neck masses in 2 to 12-year-old Iranian children presenting to the Children’s Medical Center in Tehran (which is a referral center) during a 21-year period.
2. Methods
This descriptive retrospective study was conducted on the registered pathology records of children between 2 to 12 years presenting to the Pathology department of Children’s Medical Center (which is a referral center for children in Tehran) from 1995 to 2016. Pathology records of children with head and neck masses were retrieved from the Pathology Department. Medical files with complete demographic information and pathology reports of all biopsies confirming the presence of mass with a definite diagnosis were included. The exclusion criteria were intra-orbital masses, intracranial masses and central nervous system lesions. Age, gender, type of mass, location of mass and definite histopathological diagnosis were retrieved from the medical files and recorded. The masses were classified as inflammatory/infectious/ reactive, congenital/developmental and neoplastic. Data were analyzed descriptively using SPSS version 24.
3. Results
Totally 34,188 biopsies of 2 to 12 year old children during the 21-year period at the pathology department of Children’s Medical Center were available. Of these 594 (1.75%) biopsies were of head and neck masses. The total male to female ratio was 1.7:1 (P < 0.001).
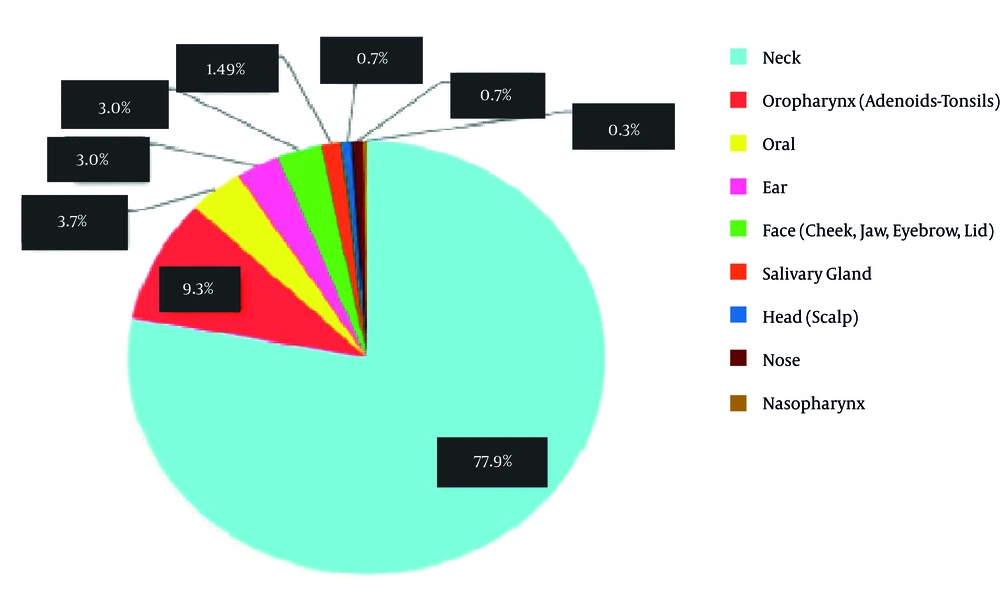
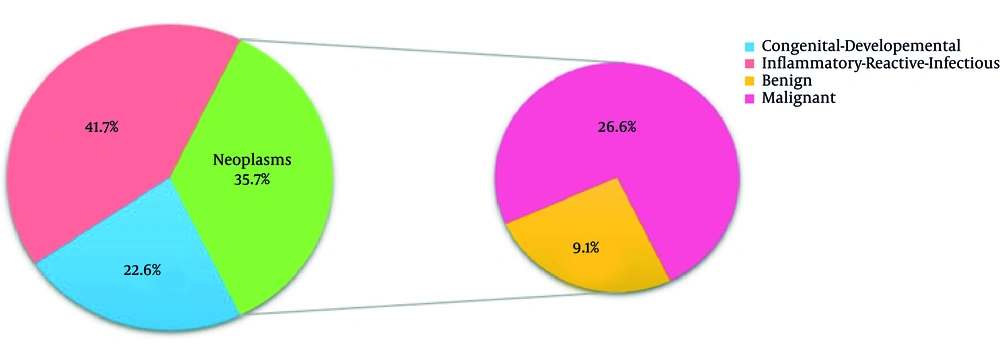
Tables 1 and 2 as well as Figure 1 show gender, age and location of all three groups. The frequency of all three groups is summarized separately in table 3-6 according to histopathologic diagnosis. As shown in Figure 1, neck was the most common site of head and neck masses (77.9%). The frequency of inflammatory/reactive/infectious lesions, neoplastic lesions and congenital/developmental lesions was 41.7%, 35.7% and 22.6%, respectively. The frequency of malignant and benign neoplastic masses was 26.6% and 9.1%, respectively (Figure 2). Figure 3 shows the distribution of masses based on their location.
| Gender | Congenital | Inflammatory | Neoplasm | Total |
|---|---|---|---|---|
| Male | 77 (13) | 165 (27.7) | 134 (22.6) | 376 (63.3) |
| Female | 57 (9.6) | 83 (14) | 78 (13.1) | 218 (36.7) |
| Total | 134 (22.6) | 248 (41.7) | 212 (35.7) | 594 (100.0) |
| M:F ratio | 1.3:1 | 2:1 | 1.7:1 | 1.7:1 |
Frequency Distribution of Different Groups of Head and Neck Masses by Gendera
| Age Group, Y | Male | Female | Total |
|---|---|---|---|
| 2.1 - 3 | 54 (9.1) | 36 (6.0) | 90 (15.1) |
| 3.1 - 4 | 42 (7.1) | 35 (5.9) | 77 (13) |
| 4.1 - 5 | 53 (8.9) | 26 (4.4) | 79 (13.3) |
| 5.1 - 6 | 48 (8.1) | 25 (4.2) | 73 (12.3) |
| 6.1 - 7 | 48 (8.1) | 19 (3.2) | 67 (11.3) |
| 7.1 - 8 | 43 (7.2) | 22 (3.7) | 65 (10.9) |
| 8.1 - 9 | 23 (3.9) | 12 (2.0) | 35 (5.9) |
| 9.1 - 10 | 25 (4.2) | 11 (1.9) | 36 (6.1) |
| 10.1 - 11 | 16 (2.7) | 16 (2.7) | 32 (5.4) |
| 11.1 -12 | 24 (4.0) | 16 (2.7) | 40 (6.7) |
| Total | 376 (63.3) | 218 (36.7) | 594 (100.0) |
Frequency Distribution of Different Age Groups by Gendera
| Diagnosis | % in All | % in Category | Frequency | Gender | Age | Sites | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | M | < 2 - < 7 | ≤ 7 - < 12 | Neck | Mouth | Face | Ear | Nose | Head | Oro pharynx | Salivary gland | Naso pharynx | ||||
| Lymphadenitis | 16.2 | 38.7 | 96 | 31 | 65 | 55 | 41 | 93 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 |
| Adenoid Hyperplasia | 9.1 | 21.8 | 54 | 15 | 39 | 33 | 21 | 0 | 0 | 0 | 0 | 0 | 0 | 54 | 0 | 0 |
| Hyperplastic Lymphoid | 7.1 | 17 | 42 | 17 | 25 | 24 | 18 | 41 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Inflammatory Process | 3.9 | 9.3 | 23 | 9 | 14 | 10 | 13 | 19 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 |
| Epidermoid Cyst | 2.7 | 6.4 | 16 | 7 | 9 | 11 | 5 | 8 | 0 | 3 | 3 | 2 | 0 | 0 | 0 | 0 |
| Ranula | 0.7 | 1.6 | 4 | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 0 |
| Abscess | 0.5 | 1.2 | 3 | 1 | 2 | 0 | 3 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Irritation Fibroma | 0.5 | 1.2 | 3 | 1 | 2 | 1 | 2 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Mucocele | 0.3 | 0.8 | 2 | 0 | 2 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 |
| Mikulicz disease | 0.2 | 0.4 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Actinomycosis | 0.2 | 0.4 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Sialadenitis | 0.2 | 0.4 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Peripheral Giant Cell Lesion | 0.2 | 0.4 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pyogenic granuloma | 0.2 | 0.4 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total | 41.7 | - | 248 | 83 | 165 | 138 | 110 | 162 | 9 | 6 | 5 | 3 | 1 | 54 | 7 | 1 |
Distribution of All Inflammatory Lesions by Gender, Age and Sitea
| Diagnosis | % in All | % in Category | Frequency | Gender | Age | Sites | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | M | < 2 - < 7 | ≤ 7 - < 12 | Neck | Mouth | Face | Ear | Nose | Head | Oro pharynx | Salivary gland | Naso pharynx | ||||
| Thyroglossal duct cyst | 11 | 48.5 | 65 | 29 | 36 | 42 | 23 | 65 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Lymphangioma | 5.2 | 23.1 | 31 | 12 | 19 | 23 | 8 | 31 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cystic higroma (71.3%) | 3.2 | 14.2 | 19 | 8 | 11 | 15 | 4 | 19 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Branchial cleft cyst | 3.7 | 16,4 | 22 | 9 | 13 | 11 | 11 | 22 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Dermoid cyst | 1.5 | 6.8 | 9 | 4 | 5 | 6 | 3 | 2 | 0 | 2 | 4 | 0 | 1 | 0 | 0 | 0 |
| Accessory Tragus | 0.8 | 3.7 | 5 | 3 | 2 | 5 | 0 | 0 | 0 | 0 | 5 | 0 | 0 | 0 | 0 | 0 |
| Ectopic cervical thymus | 0.3 | 1.5 | 2 | 0 | 2 | 1 | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total | 22.6 | 134 | 57 | 77 | 88 | 46 | 122 | 0 | 2 | 9 | 0 | 1 | 0 | 0 | 0 | |
Distribution of All Congenital Lesions by Gender, Age and Sitea
| Diagnosis | % in All | % in Category | Frequency | Gender | Age | Sites | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | M | < 2 - < 7 | ≤ 7 - < 12 | Neck | Mouth | Face | Ear | Nose | Head | Oro pharynx | Salivary gland | Naso pharynx | ||||
| Lymphoma | 22.4 | 84.2 | 133 | 34 | 99 | 45 | 88 | 133 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hodgkin (72.9%) | 16.3 | 61.4 | 97 | 25 | 72 | 31 | 66 | 97 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Non-Hodgkin (27.1%) | 6.1 | 22.8 | 36 | 9 | 27 | 14 | 22 | 36 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Rhobdomyosarcoma | 1.3 | 5.1 | 8 | 5 | 3 | 3 | 6 | 8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Neuroblastoma | 1 | 3.2 | 6 | 5 | 1 | 3 | 3 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Leukemia | 0.7 | 2.6 | 4 | 0 | 4 | 0 | 4 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Undifferentiated Carcinoma | 0.3 | 1.3 | 2 | 0 | 2 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| Papillary Thyroid Carcinoma | 0.3 | 1.3 | 2 | 1 | 1 | 1 | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Metastatic Papillary Thyroid Carcinoma | 0.2 | 0.7 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Angiosarcoma | 0.2 | 0.7 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Leiomyosarcoma | 0.2 | 0.7 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total | 26.6 | - | 158 | 47 | 111 | 55 | 103 | 155 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 |
Distribution of All Malignant Neoplastic Lesions by Gender, Age and Sitea
| Diagnosis | % in All | % in Category | Frequency | Gender | Age | Sites | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | M | < 2- < 7 | ≤ 7- < 12 | Neck | Mouth | Face | Ear | Nose | Head | Oro pharynx | Salivary gland | Naso pharynx | ||||
| Pilomatrixoma | 3.5 | 38.9 | 21 | 11 | 10 | 15 | 6 | 12 | 0 | 5 | 4 | 0 | 0 | 0 | 0 | 0 |
| Hemangioma | 2.9 | 31.5 | 17 | 10 | 7 | 12 | 5 | 1 | 11 | 2 | 0 | 1 | 2 | 0 | 0 | 0 |
| Capillary Hemangioma | 1 | 11.1 | 6 | 3 | 3 | 6 | 0 | 0 | 3 | 2 | 0 | 0 | 1 | 0 | 0 | 0 |
| Fibromatosis | 0.5 | 5.6 | 3 | 2 | 1 | 2 | 1 | 2 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Thyroid Nodule | 0.5 | 5.6 | 3 | 3 | 0 | 0 | 3 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Granular Cell Tumor | 0.5 | 5.6 | 3 | 3 | 0 | 3 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Lipoma | 0.3 | 3.7 | 2 | 1 | 1 | 2 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Neurofibroma | 0.3 | 3.7 | 2 | 0 | 2 | 2 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Thyroid Adenoma | 0.2 | 1.8 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Inflammatory Myofibroblastic tumor | 0.2 | 1.8 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Leiomyoma | 0.2 | 1.8 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total | 9.1 | - | 54 | 31 | 23 | 37 | 17 | 24 | 13 | 9 | 4 | 1 | 2 | 0 | 1 | 0 |
Distribution of All Benign Neoplastic Lesions by Gender, Age and Sitea
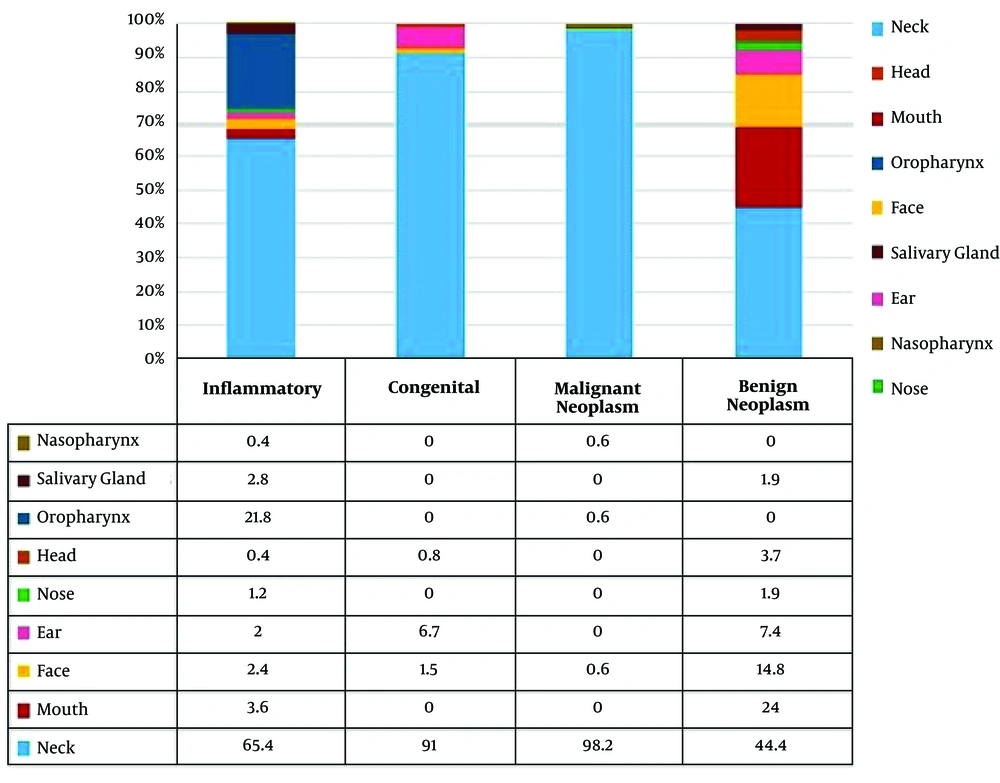
Tables 3 - 6 show masses based on age, gender and location of lesion. In the category of inflammatory/reactive/infectious lesions, lymphadenitis (38.7%), adenoid hyperplasia (21.8%) and lymphoid hyperplasia (17%) were among the most common lesions. The male to female ratio was 2:1 (Table 3). In the category of congenital lesions, thyroglossal cyst (48.5%) (about half of the congenital lesions), lymphangioma (cystic hygroma) (23.1%), branchial cleft cyst (16.4%) and dermoid cyst (6.8%) were the most common types (Table 4). Male to female ratio in all congenital lesions was 1.3:1.
In the category of malignant neoplastic lesions, the most common lesions were lymphoma (84.2%; Hodgkin lymphoma 61.4% and non-Hodgkin lymphoma 22.8%), rhabdomyosarcoma (5.1%) and neuroblastoma (3.2%) (Table 5). Four cases of leukemia were detected. The patients in whom already leukemia was diagnosed, presented with a mass. In general, the prevalence of malignant lesions in males was higher than that in females (2.4:1). Except for lymphoma, the other malignant lesions were slightly more common in females, and male to female ratio was 0.9:1. The most common benign lesions were pilomatrixoma (38.9%), hemangioma (31.5%), fibromatosis, thyroid nodule and granular cell tumor (5.6% each) (Table 6). All benign lesions were more prevalent in females than in males (male to female ratio of 0.7:1).
4. Discussion
The present study aimed to show the frequency of head and neck masses in 2 to 12-year-old Iranian children presenting to the Children’s Medical Center in Tehran during a 21-year period. In this study we tried to answer following questions: What is the frequency of the lesions in 2 to 12-year-old Iranian children? What is the most common lesion in head and neck region in children? What is the sex predilection? Among the inflammatory/infectious/reactive, congenital/developmental and neoplastic which one is the most common? What is the most common lesion in each group? Where is the most common site in each group?
The prevalence of these lesions in 2 to 12 year old children was 1.75%. Males were more affected than females (1.7:1). Although inflammatory/reactive/infectious lesions were the most common masses in the head and neck region in children, lymphoma was the most prevalent one among all lesions. Neck was the most common site of head and neck masses.
The prevalence of inflammatory/reactive/infectious lesions was also the highest in our study (41.7%). In line with our results, many other studies have reported that inflammatory lesions have the highest frequency among the head and neck masses in children; this frequency varied from 43.9% in the study by Lucumay (12) to 58% in the study by Al-Mayoof (13). However, some studies have reported that inflammatory lesions rank second or third in terms of frequency and prevalence (20% to 33.5%) (14-16). In general, the highest prevalence of inflammatory lesions was 57.8% reported by Al-Mayoof (13), in Baghdad and the lowest prevalence rate was 20% reported by Osifo and Ugiagbe in Nigeria (15). In our study, inflammatory/reactive/infectious lesions showed a significantly higher frequency in males (2:1). The mean age reported in our study (6.4 years) was slightly lower than the overall mean age (6.5 years).
In our study, neoplastic lesions ranked second (n = 212, 35.7%) in terms of prevalence, which was in contrast to the findings of some other studies. Head and neck neoplasms occur in children in the age range of one day to 16 years; the highest frequency was reported by Ayugi in Kenya (26.8%) (6). Also, in our study, the male to female ratio was 1.7:1 in the neoplastic group and the mean age was seven years, which was higher than the overall mean age (6.5 years).
In this study, 74.5% of neoplasms were malignant and the remaining 25.5% were benign. In two studies conducted in India and Kenya, and in our study, benign masses were less frequent than malignant masses (4, 6); whereas, in many studies, in contrast to ours, this order was reverse and the frequency of benign tumors was much higher than malignant tumors. The frequency of benign tumors relative to all tumors has been variable from 68 to 77.8% (10, 12, 16, 17). In a study conducted on cervical masses in children in China, benign lesions were more than three times more common than malignant lesions (16). In general, the lowest prevalence of malignancies among neoplastic lesions was noted in two studies conducted in China (16) (22.2%) and Saudi Arabia (25%) (18). The highest prevalence of malignancies is reported in three studies conducted in Baghdad (87.2%), Kenya (88%) and Shiraz (89.5%) (6, 13, 19). Of 10 studies evaluated, the results of studies by Al-Mayoof in Baghdad (87.2%) were the closest to ours (4, 6, 10, 12-14, 16, 19).
In our study, about 0.5% of pathological lesions in 2 to 12 year olds were malignancies in the head and neck region. Of 594 masses, 158 (26.6%) were malignant and ranked second after inflammatory/infectious/reactive lesions in terms of prevalence. Malignant lesions were more common in males (2.4:1 ratio). Also, the highest mean age (7.7) belonged to the malignant lesions. The overall percentage of malignancies in studies on the head and neck masses in children ranged from 2.6% in China to 11.7% in Turkey (4, 13, 14, 16, 18). In contrast, Osifo and Ugiagbe in their study on cervical masses in children in Nigeria reported different statistics. In their study, malignant lesions ranked in the first (57%), congenital lesions in the second (23%) and inflammatory lesions in the third (20%) place (15). A study carried out by Ayugi et al. in Kenya reported the closest values to our study in terms of frequency of malignancies and head and neck masses. At that study the overall prevalence of malignancies was 32% (6).
Benign lesions (n = 54; 9.1%) ranked last in terms of prevalence in our study. They were more common in females (male to female ratio 0.7:1). Also, the mean age of patients with benign lesions was 5.9 years, which was lower than the overall mean age.
Congenital/developmental lesions had a prevalence of 22.6% and ranked third in terms of prevalence in our study. In two studies conducted in Turkey and China, these lesions were the most common head and neck lesions with over 50% prevalence rate (14, 16). In most previous studies, congenital lesions ranked second in terms of prevalence (4, 12, 13). However, in the study by Ayugi (20) and our study, congenital lesions of the neck ranked third with 22% prevalence rate. In two other studies on cervical masses, the frequency of congenital lesions ranged from 12% to 18.9% irrespective of age (21, 22). In our study, congenital/developmental lesions were slightly more common in males (1.3:1), and had a lower mean age (5.9 years) than the overall mean age. In a study by Shengwei in China, congenital lesions were more common in males (male to female ratio of 1.4:1) (16). The most common lesions in our study were lymphoma (22.4%), lymphadenitis (16.2%) and thyroglossal cyst (10.9%). Lymphomas account for over 50% of malignancies in the head and neck region in children and are mainly seen in the form of cervical masses (23). In many studies, similar to our study, lymphomas were the most common masses found. In these studies, the prevalence of lymphoma ranged from 23.8% to 88.2% (5, 6, 10, 12-19, 24-27). Another important issue is that the incidence of lymphoma in other studies was similar to that in our study and the closest rate was 83.3% reported in a study conducted in Tanzania (12-15, 25).
In general, cervical lymphadenitis is very common in children. In children, most lymphadenopathies are related to infections (28). In our study, lymphadenitis with 38.7% prevalence rate was the most common inflammatory/reactive/infectious mass of the head and neck region; of which, 28.2% were reactive lymphadenitis and 6.4% were lymphadenitis due to tuberculosis. Of four studies on the frequency of inflammatory masses of the head and neck region in children, lymphadenitis was the most common lesion in five studies in addition to ours (4, 6, 13, 16). In a study conducted in Kenya, similar to our study, reactive lymphadenitis (29.1%) and lymphadenitis due to tuberculosis (21.4%) were the most common types (6). In the afore-mentioned six studies, the frequency of lymphadenitis among inflammatory masses ranged from 12.3% in Tanzania to 94.5% in China (4, 6, 12-16). Another study conducted in 2014 in Turkey reported a prevalence rate of 42.9%, which was the closest to our result (14). In many studies on cervical congenital masses, thyroglossal cyst was the most common after cervical lymphadenopathy accounting for 70% of all congenital masses. Also, it was the most common mass occurring at the cervical midline in children (29-31). In many previous studies, as well as our study, thyroglossal cyst was the most common (20, 21, 24-27, 29). In two other studies conducted in Tanzania and China, thyroglossal cyst was not the most common but was among the prevalent cysts (25, 28). In contrast, a study conducted in 2002 in India did not report thyroglossal cyst among the masses (4). This difference may be due to assessment of a short period of time (one year).
In this study the frequency of the head and neck lesions in male patients was higher than in females (1.7:1). This was very similar to studies carried out by Ragesh et al., Al-Mayoof and Osifo and Ugiagbe (4, 24, 27) and was different from other studies. In the studies with different results, only congenital lesions were evaluated (20, 21).
In conclusion, head and neck masses have a prevalence of less than 2% in children. Most of these masses are benign. However, their high prevalence should not be neglected. Except for benign neoplastic masses, head and neck masses are more common in males. Malignant neoplastic lesions less commonly occur in very young age; but inflammatory and congenital masses have a higher prevalence at a younger age. Also, neck is the most commonly involved site.
This study was carried out based on pathologic records. The limitation of the study was missing of some demographic data in pathologic records. On the other hand, the lesions without definite diagnosis were reviewed by pathologist again. Considering the significance of this topic, further studies are required on head and neck masses in children. Also, signs and symptoms and patients’ clinical condition at the time of presentation, primary diagnosis, diagnostic workup performed and the treatment plan of these patients should be discussed in future studies.