1. Background
Progressive familial intrahepatic cholestasis (PFIC), also known as Byler disease, has been described as a group of childhood autosomal recessive disorders which present with hepatocellular cholestasis (1, 2). The disease was originally described in an Amish child who presented with recurrent bouts of jaundice, associated with light stools, dark urine and pruritis (3). It has been reported as a rare cause of childhood cholestasis and cirrhosis in Iran (4-6).
Three genotypes of PFIC have been described and associated with mutations in hepatocellular transport system genes (PFIC1, 2, 3) (2). PFIC1 is formed secondary to a mutation in ATP8B1 that codes an amino phospholipid transporter, PFIC2 is formed secondary to a mutation in ABCB11 that codes the bile salt export protein, and PFIC3 is caused by a mutation in the ABCB4 gene that codes the canalicular phospholipid export pump (MDR3). These genotypes have different clinical, biochemical and genetic characteristics. Patients suffering from PFIC1 and 2 present in the neonatal period or early infancy, as it appears in the first months of life; meanwhile, PFIC3 may arise later in infancy, in childhood or even during young adulthood (7, 8).
Diagnosis is usually made by using a combined clinical, biochemical, radiological, histological and genetic approach, together with liver immunostaining and biliary lipid analysis (9, 10). Serum GGT (gamma glutamine transpeptidase) is normal in patients with PFIC1 and PFIC2, whereas those with PFIC3 have high serum GGT activity (11).
Electron microscopy of the bile in PFIC1 shows coarse and granular (Byler’s) bile in comparison to PFIC2 and 3, which show amorphous bile (12-14).
Liver histologic studies are of considerable importance when evaluating a patient for PFIC since they allow for immunostaining to be performed. A number of commercially available MDR3 (multi-drug resistance 3) and BSEP (bile salt export pump) antibodies have been used (7, 8, 12). In immunohistochemistry studies, PFIC3 is associated with mild or absent canalicular staining with MDR3 (due to a mutation in the ABCB4 gene that codes the canalicular phospholipid export pump, MDR3), while PFIC2 shows similar findings with regard to BSEP antibodies (due to a mutation in ABCB11 that codes the bile salt export protein) (13).
Genetic testing involves DNA sequencing the 27 coding exons and their splice junctions. A resequencing chip, dedicated to looking for the genetic syndromes of cholestasis, has been developed and may facilitate diagnosis. As no phenotypic features can exclude PFIC1 or 2 in a patient with normal GGT PFIC, immunohistochemistry with BSEP staining followed by genetic analysis is recommended. In patients with negative BSEP staining, one should first test for ABCB11, whereas in patients with normal BSEP staining, ATB8B1 mutation should be considered (12-14).
2. Objectives
To the best of our knowledge, there has yet to be a reported study classifying PFIC genotypes in Iran. In this four-year cross-sectional study (2010 - 2014), we aimed to determine and evaluate the PFIC genotypes in Iranian patients by using immunohistologic staining for 68 patients with confirmed PFIC.
3. Methods
The present study was designed as a cross-sectional analysis on the explanted livers and needle biopsy specimens of 68 patients with a confirmed PFIC diagnosis over a period of four years (June 2010 - June 2014) in Namazi hospital, which is affiliated with the Shiraz University of Medical Sciences and is the largest referral center of liver diseases in Southern Iran.
Baseline demographics, including patient age, gender, liver function tests and clinical symptoms, were collected from the clinical charts and recorded.
Paraffin-embedded blocks of explanted liver tissue and the liver biopsies of PFIC patients were extracted from the archives of pathology and immunostaining was performed based on standard immunohistochemical procedures to detect canalicular proteins.
IHC was performed by routine method, i.e., a 4 - 5µm section of the proper paraffin block was prepared for each case; then, after deparaffinization in xylol and hydration with 100%, 96% and 70% alcohol and washing steps with distilled water and phosphate buffer saline (PBS) and adding H2O2, antigen retrieval was performed. Overnight, secondary antibodies were applied and counterstaining with hematoxylin was the final step.
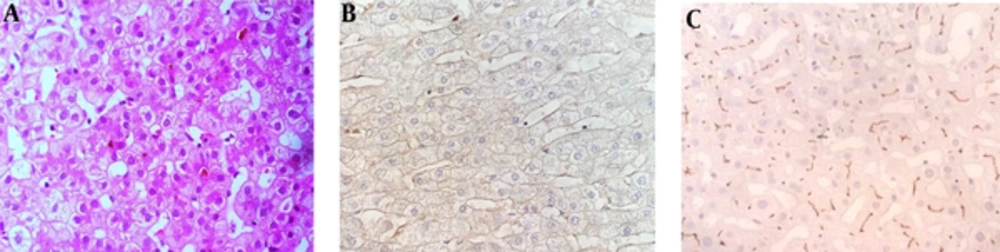
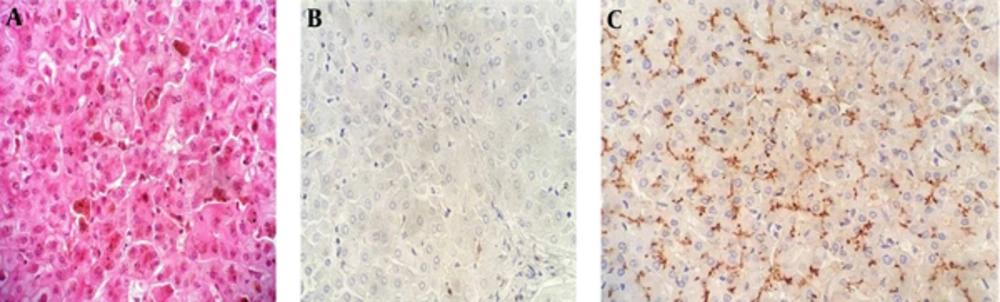
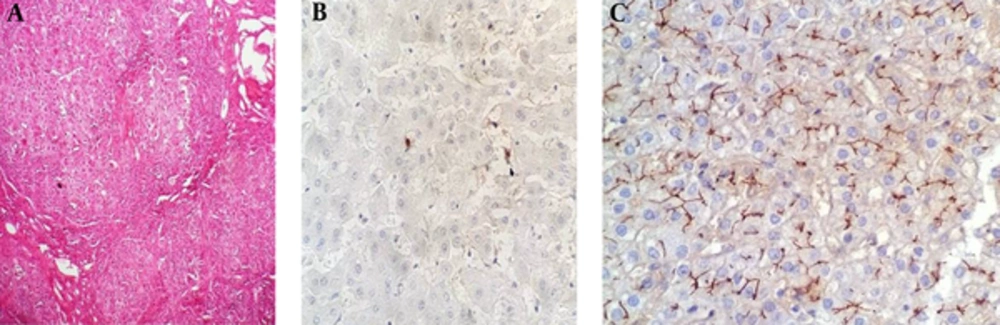
The following antibodies were used: anti-ATP8B1 and CD10, in order to investigate PFIC1, anti-ABCB11 (anti-BSEP) for PFIC type 2, and anti-ABCB4 (MDR3 protein) for PFIC type 3. Table 1 shows the characteristics of the antibodies. Immunostaining was classified as positive (when bile canaliculi were stained) and negative (when bile canaliculi were not stained).
| Antibody | Company | Dilution | Antigen Retrieval |
|---|---|---|---|
| ATP8B1 | Abcam | 1/200 | Tris-EDTA |
| ABCB11 | Abcam | 1/500 | Tris-EDTA |
| ABCB4 | Abcam | 1/200 | Tris-EDTA |
Normal liver tissue staining with the above antibodies was considered the positive control for all three types.
4. Results
Sixty-eight patients with a confirmed PFIC diagnosis were investigated by immunostaining. Forty-three (63.2%) of the cases were male and the remaining twenty-five cases (36.8%) were female.
Among these 68 specimens, 18 (26.5%) were liver biopsies and 50 (73.5%) specimens were explanted livers.
The baseline clinical and biochemical characteristics of the studied patients are shown in Table 2.
| Variables | Mean ± SD | Minimum-Maximum |
|---|---|---|
| Age, y | 4.8± 4.5 | 3 months - 19 years |
| GGT Level, IU/L | 94.9 ± 143.2 | 6 - 350 |
| AST, IU/L | 163.3 ± 237 | 28 - 1050 |
| ALT IU/L | 177.7 ± 293 | 13 - 1440 |
| Total Bilirubin, mg/dL | 6.9 ± 10.8 | 30 - 60.50 |
| Direct Bilirubin, mg/dL | 3.2 ± 4.5 | 10 - 21.60 |
| Total Protein, g/dL | 6.5 ± 1.4 | 2.5 - 10 |
| Serum Albumin, g/dL | 3.6 ± 0.5 | 2.1 - 4.7 |
aNormal levels: ALT < 40 IU/L, AST normal < 40IU/L, GGT < 45IU/L.
The majority (n = 34) of cases were PFIC type 2 (50%) and 29 cases were type 1 (42.6%). The rest of the cases (n = 5, 7.4%) were categorized as type 3. The most common presenting symptoms were jaundice (82.4%) and pruritis (48.5%) followed by vomiting (7.4%) and bleeding tendency (4.45%).
4.1. PFIC Type 1
Twenty-nine (42.6%) cases had normal IHC studies using anti-ABCB4 and anti-ABCB11 antibodies and abnormal (not stained) ATP8B1 and CD10 antibodies which was in favor of PFIC type 1 (Figure 1). Seventeen patients (58.6%) were male and twelve patients (41.4%) were female. Tables 3 and 4 summarize the baseline characteristics of PFIC type 1 patients.
| Variables | Mean ± SD | Minimum-Maximum |
|---|---|---|
| Age years | 5.1 ± 4.8 | 3 months - 18 years |
| GGT Level | 112.6 ± 16.5 | 9 - 74 |
| AST, U/L | 168.2 ± 228.2 | 28 - 940 |
| ALT, U/L | 167.9 ± 289.1 | 13 - 1350 |
| Total bilirubin, mg/dL | 6.5 ± 8.8 | 0.3 - 43.0 |
| Direct bilirubin, mg/dL | 3.1 ±3.8 | 0.1 - 17.3 |
| Serum albumin, g/dL | 3.6 ± 0.5 | 2.5 - 4.6 |
| Total protein, g/dL | 6.4 ± 1.5 | 2.8 - 10.0 |
aNormal levels: ALT < 40 IU/L, AST normal < 40IU/L, GGT < 45IU/L.
| Presenting Symptoms | No. (%) |
|---|---|
| Jaundice | 24 (82.8) |
| Pruritis | 14 (48.3) |
| Bleeding tendency | 1 (3.4) |
| Vomiting | 2 (6.9) |
| Poor feeding | 2 (6.9) |
4.2. PFIC Type 2
Absence of canalicular immunostaining with anti-ABCB11 antibody was evident in 34 (50%) cases (Figure 2). Twenty-two (64.7%) patients were male and twelve (35.3%) patients were female. Tables 5 and 6 summarize the baseline characteristics of patients with PFIC type 2.
| Variables | Mean± SD | Minimum-Maximum |
|---|---|---|
| Age, y | 4.4 ± 4.7 | 7 months - 19 years |
| GGT, IU/L | 68.1 ± 11.8 | 6.0 - 60 |
| AST, IU/L | 152.1 ± 257.7 | 36.0 - 1050.0 |
| ALT, IU/L | 165.1 ± 307.8 | 17 - 1440 |
| Total Bilirubin, mg/dL | 7.6 ± 13.1 | 0.3 - 60.5 |
| Direct Bilirubin, mg/dL | 3.5±5.2 | 0.1 - 21.6 |
| Serum Albumin, g/dL | 3.7 ± 0.6 | 2.1 - 4.7 |
| Total Protein, g/dL | 6.6 ± 1.3 | 2.5 - 8.9 |
aNormal levels: ALT < 40 IU/L, AST normal < 40 IU/L, GGT < 45 IU/L.
| Presenting Symptom | No. (%) |
|---|---|
| Jaundice | 27 (79.4) |
| Pruritis | 18 (52.9) |
| Bleeding tendency | 1 (2.9) |
| Vomiting | 2 (5.9) |
4.3. PFIC Type 3
Five (7.4%) patients were categorized as type 3, all of which were discovered in cirrhotic explanted livers (Figure 3). Four (80%) of the cases were male and one case (20%) was female. No type 3 cases were found in patients less than one year of age. The demographics, symptoms, clinical findings and biochemical data for a total of five patients with PFIC 3 are summarized in Tables 7 and 8.
| Variables | Mean ± SD | Minimum-Maximum |
|---|---|---|
| Age y | 6.2 ± 1.3 | 4 - 7 years |
| GGT Level, IU/L | 179.0 ± 153.2 | 19 - 350 |
| AST, IU/L | 211.2 ± 170.2 | 35 - 479 |
| ALT, IU/L | 317.2 ± 224.9 | 33 - 514 |
| Total Bilirubin, mg/dL | 4.1 ± 4.2 | 0.4 - 10.6 |
| Direct Bilirubin, mg/dL | 2.5 ± 2.8 | 0.1 - 7.1 |
| Serum Albumin, g/dL | 3.6 ± 0.6 | 3.0 - 4.5 |
| Total Protein, g/dL | 6.2 ± 0.7 | 5.4 - 6.9 |
aNormal levels: ALT < 40 IU/L, AST normal < 40 IU/L, GGT < 45 IU/L.
| Presenting Symptom | No. (%) |
|---|---|
| Jaundice | 5 (100.0) |
| Pruritis | 1 (20.0) |
| Bleeding Tendency | 1 (20.0) |
| Vomiting | 1 (20.0) |
5. Discussion
Progressive familial intrahepatic cholestatic (PFIC) disease constitutes between 10% - 15% of the causes of cholestasis in pediatric patients and this disease is the cause of 10% - 15% of liver transplants (15). It should be accurately differentiated from other cholestatic liver disorders in children because of the differences in its prognosis and treatment (7).
PFIC typing is also important as the course of the disease, some clinical and extrahepatic manifestations and paraclinical tests differ according to the three types of the disease (7).
Immunohistochemical study is a helpful method to diagnose and classify the disease (10, 12, 14-16); however, there has not yet been such a study regarding the classification of the patients with PFIC in the Iranian population.
As indicated in the previous studies, there are three different antibodies used in the IHC method to type the PFIC disease: anti-ATP8B1 and anti-CD10 are used for type 1, anti-BSEP (ABCB11) is used for type 2 and anti-MDR3 (ABCB4) is used for type 3 of the disease (10, 12, 14-20).
A powerful correlation has been described between the antibodies detecting the ultimate product of each type of the disease with specific mutations (10, 12, 14-16, 19).
In our population, the most frequent types were type 2 (50%) and type 1 (42.6%), with only five (7.4%) of the cases being considered type 3. These results are compatible with the results of the previous literature that studied the three types simultaneously. For instance, Giovannoni et al. (21) studied 27 Italian patients, both by gene study and immunohistochemistry, and found that type 2 of the disease is the most frequent (17 patients = 63%), followed by type 1 (7 = 26%) and type 3 (3 = 11%). In 62 French patients studied by Davit-Spraul, 39 (63%) cases were type 2, 13 (21%) cases were type 1 and 10 (16%) of the cases were categorized as unknown (8).
Many of the authors of past studies have concentrated on only one type of the disease, while more recent studies have shown different methods for subtyping all 3 types of PFIC. Table 9 shows the results of different studies from several countries regarding the frequencies of different types of PFIC using either gene studies or immunohistochemistry. According to the clinical presentation, all three types generally presented with jaundice and pruritis; however, GGT levels were higher in type 3, which aligns with previous reports (8).
| Author | Country | Year | Method of Typing | Total Patients | Type 1 No. (%) | Type 2 No. (%) | Type 3 No. (%) |
|---|---|---|---|---|---|---|---|
| Giovannoni I et al. (21) | Italy | 2015 | IHC/Gene study | 27 | 7 (26) | 17 (63) | 3 (11) |
| Davit-Spraul et al. (8) | France | 2010 | IHC/Gene study | 62 | 13 (21) | 39 (63) | |
| Klomp et al. (22) | Netherlands | 2004 | Gene study | 180 | 54 (30) | ||
| Evason et al. (16) | USA | 2011 | IHC/Gene study | 12 | 10 (83) | ||
| Strautnieks et al. (12) | England | 2008 | 88 | 82 (93) | |||
| Chen et al. (23) | Taiwan | 2008 | IHC/HPLC/SEQUENCIG | 18 | 4 (25) | ||
| Chen et al. (24) | Taiwan | 2001 | Gene study | 47 | 1 (2) | ||
| Colombo et al. (25) | Italy | 2011 | Gene study | 133 | 44 (33) | ||
| Jacquemi et al. (10) | France | 2001 | IHC/Gene study | 12 | 9 (75) | ||
| El-Guindi et al. (26) | Egypt | 2016 | IHC | 25 | 2 (8) | 17 (68) | 6 (24) |
| Our study | Iran | 2016 | IHC | 68 | 29 (42.6) | 34 (50) | 5 (7.4) |
5.1. Conclusions
PFIC can be accurately typed by IHC studies. In our center, we found type 2 to be the most common, followed by types 1 and 3. This is similar to the frequencies reported in the West.