1. Background
Hyperbilirubinemia is a common condition, which usually resolves during two weeks after birth. infant cholestasis (IC) is defined as prolonged conjugated hyperbilirubinemia which results from diminished bile flow and/or excretion with different etiologies (1). Infant cholestasis is a serious condition that requires immediate investigation because it may be due to life-threatening events such as biliary atresia that needs prompt intervention. Therefore, the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition cholestasis guideline recommends measurement of total and direct serum bilirubin in any jaundiced infant with prolonged jaundice at two weeks of age and any healthy breast-fed infants at three weeks of age (2).
Investigation of infants with cholestasis is difficult due to various etiologies. The two most common causes of infant cholestasis are biliary atresia (BA) and idiopathic neonatal hepatitis (INH) (3). Diagnosis of biliary atresia from other causes of liver injury is still a challenging phenomenon due to high overlap in clinical presentation as well as laboratory findings, imaging, and histological pictures. Of course, early diagnosis is particularly important in subjects with biliary atresia because early surgical treatment with Kasai portoenterostomy improves outcome and survival (4). Liver biopsy often remains the cornerstone of the diagnostic workup in infant cholestasis with a diagnostic yield as high as 90% to 95% by an experienced pathologist (5).
Up to now, no report on infant cholestasis exists from our region, so we analyzed retrospectively infants with cholestasis who were referred to a single tertiary center during a 6-year period. The aim was to report the frequency of different causes of infant cholestasis diagnosed by clinical and biochemical parameters as well as liver biopsy in a tertiary referral center from South-East Iran.
2. Methods
Medical records of 46 cholestatic infants during a 6 year period between 2011 and 2017 were retrospectively reviewed. Subjects were clinically investigated for infant cholestasis in a teaching referral center in Ali-Ebne-Abitaleb Hospital, Zahedan, Iran which mostly receives patients from different areas of Sistan-Baluchestan province with an area of 181,785 km2 and a population of 2.7 million with 19 cities, located at South-East of Iran.
Cholestasis persisting beyond two weeks of age in infants less than 6 months old were studied with complete clinical data, and results of liver pathology. Subjects with incomplete data in their medical record were excluded from the study. The studied 46 infants had cholestasis with conjugated bilirubin > 1mg / dL and total bilirubin < 5 mg / dL or when it comprised more than 20% of the total bilirubin if total bilirubin was > 5 mg / dL (6).
Information on age, gender, birth weight, onset and duration of jaundice, clay color stool, hepatomegaly and splenomegaly, abdominal ultrasonography, Tc99m-hepatobiliary iminodiacetic acid (HIDA) scintigraphy, and results of percutaneous liver biopsy were recorded. Also, laboratory investigations including Liver function tests, total and direct serum bilirubin, alkaline phosphatase (ALP), gamma glutamyle transferase (GGT), blood glucose, urine examination and reducing substances, complete blood count (CBC), serological tests for hepatitis B, C and TORCH antibodies, sweat chloride test and thyroid function tests were done. Performance of serum alpha-1-antitrypsin was not available in our laboratory. Results of liver biopsy and pathology findings were recorded. An informed consent was obtained from the parents of all patients.
One infant was considered to have idiopathic neonatal hepatitis after a thorough history taking, physical examination and laboratory evaluation failed to identify the underlying cause of the infant cholestasis. Also, after performing percutaneous liver biopsy, final diagnosis of biliary atresia in infants with cholestasis was based on laparotomy and intraoperative cholangiogram. According to the diagnosis, patients were classified into two groups: Group 1 as biliary atresia (BA) and group 2 as non-biliary atresia (Non-BA) including idiopathic neonatal hepatitis, cirrhosis, CMV, progressive familial intrahepatic cholestasis (PFIC) and metabolic cases. Results were expressed as mean ± SD. Chi-square test and Student’s t test were used for comparison and P value < 0.05 was taken as significant.
3. Results
Data of 46 infants with infant cholestasis in the study period, who had liver biopsy, was analyzed. There were 25 (54.3%) girls and 21 (45.7%) boys with the mean age of 65 ± 99 days. The birth weight of the patients was 1600 - 3800 gram. Fifty-two percent of the patients had jaundice in the first week after birth. Time of referral varied among subjects. Most of infants (19 cases, 42%) were referred at the age of > 91 days, and only 2 (4%) cases were less than 30 days old. Most common symptoms and signs were jaundice in 100% followed by hepatomegaly (78%), splenomegaly (52%), acholic stool (35%) and dark urine (37%). Ultrasonography was helpful in diagnosing extra hepatic BA in 9 cases. HIDA scan was performed in 18 patients who revealed neonatal hepatitis in 8 (17.4%) cases, biliary atresia in 4 (9%) cases, 2 (4%) cases had normal findings and 4 cases equivocal findings. Clinical and laboratory findings of biliary atresia and non-biliary atresia groups at presentation are shown in Table 1.
| Parameter | Biliary Atresia Cases (n = 14) | Non-Biliary Atresia Cases (n = 32) | P Value |
|---|---|---|---|
| Age (days) | 52 ± 102 | 71 ± 98 | 0.80 |
| Onset of jaundice (days) | 36 ± 25 | 10.3 ± 10.2 | 0.10 |
| Female / male | 10 / 4 | 15 / 17 | 0.10 |
| Jaundice | 14 (100%) | 31 (96%) | 0.50 |
| Acholic stool | 9 (64%) | 7 (25%) | 0.01 |
| Hepatomegaly | 12 (85%) | 24 (80%) | 0.60 |
| Splenomegaly | 10 (71%) | 14 (50%) | 0.10 |
| Cataract | 0 | 5 (38.5%) | 0.03 |
| Total bilirubin (mg / dl) | 4.5 ± 8.8 | 6.6 ± 12.7 | 0.06 |
| Direct bilirubin (mg / dl) | 2.8 ± 6 | 4.5 ± 7 | 0.40 |
| AST (IU / L) | 142 ± 367 | 137 ± 234 | 0.01 |
| ALT (IU / L) | 125 ± 216 | 86 ± 122 | 0.005 |
| Alkaline phosphatase (IU / L) | 237 ± 1394 | 1752 ± 1923 | 0.60 |
| PT (sec) | 15 ± 7.5 | 12.7 ± 1.4 | 0.20 |
Clinical and Laboratory Findings of Biliary Atresia and Non-Biliary Atresia Groups at Presentation
There were no significant differences between two groups with regard to variables such as age, gender, jaundice, onset of jaundice, hepatomegaly and splenomegaly. However, a significant difference was observed between some variables. Onset of jaundice was earlier in non-BA cases. Patients in biliary atresia group had more acholic stools, abnormal AST, ALT and total bilirubin (P < 0.05). Children in BA group experienced prolonged prothrombin time (PT) due to severe cholestasis and late referral, although it was not significant (P = 0.2). Cases with severe cholestasis and prolonged prothrombin time received vitamin K and FFP. Infants in non-biliary atresia group had cataract in 5 cases reducing substance in urine was positive in 4 (8.7%) cases. Positive galactose-1-phosphate uridyl transferase assay was suggestive of galactosaemia in 10.9%. For all other biochemical parameters there were no significant differences between the two groups.
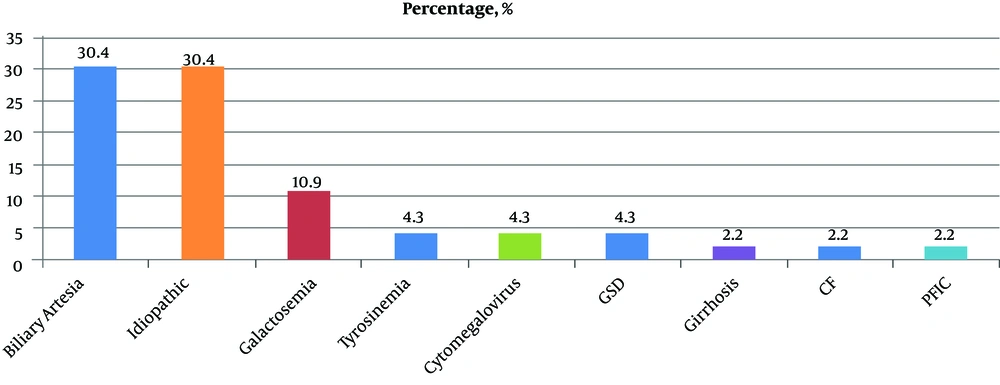
The most common underlying causes of liver disease in study group were biliary atresia (n = 14) and idiopathic neonatal hepatitis (INH) (n = 14) which was detected by liver biopsy. Also, other tests such as galactose-1-phosphate uridyl transferase assay, eye examination, succinylacetone quantification and sweat test revealed metabolic disorders, namely galactosemia, tyrosinemia and cystic fibrosis. Frequency of infant cholestasis in study group is shown in Figure 1.
4. Discussion
Infants with cholestasis should be evaluated promptly for potentially life-threatening and treatable causes whereby timing of intervention directly impacts clinical outcomes (7). Results of this study showed that most (60.8%) cholestatic infants had BA and INH. Also, majority of referred cases were older than three months.
Findings of the present study are in accordance with the literature concerning the etiology of infant cholestasis (8, 9). In comparison with previous reports, the proportion of our BA cases (34%) seems to be almost similar to other studies (8, 10). Of course, one important aspect of cholestasis in infant is to differentiate BA from other causes since the success of Kasai portoenterostomy is associated with the age at the time of surgery (10). In a study on 743 infants with BA, survival rates with native liver decreased as the age at surgery increased from less than 45 to 90 days. The investigators estimated that if patients with BA underwent Kasai operation before 46 days of age, 5.7% of all liver transplantations performed annually could be avoided (11). Considerable delay in the referral of patients with biliary atresia was documented in present study. Also, a significant delay in presentation of cholestasis and liver biopsy in a research from Iran was found which may have caused irreversible liver damage in conditions such as BA (12). Such a late referral would affect not only the success rate of surgery, but also its outcomes. New researches have shown that advanced age and degree of hepatic fibrosis are strongly correlated with poor outcomes following portoenterostomy (13). Therefore, efforts to train physicians as well as promoting public awareness of delayed referral should be highly recommended.
Another noticeable finding was detecting INH (30.4 %) as an important cause of intrahepatic cholestasis. In contrast to a study in Germany (10), the percentage of infants diagnosed with idiopathic cause was higher (30.4% vs. 13%). Also, metabolic causes of IC were found in 22% of cases with galactosemia as the most finding problem which is in line with study by Rafeey et al. (14) and is different from other studies. An explanation for this discrepancy could be that some cases might have been missed because of non-availability of new diagnostic tools such as some molecular genetic tests, e.g. alpha1-antitrypsin deficiency (AATD), in our area, which is one of our limitations. But in spite of all restrictions, we diagnosed galactosemia in 11% of children, which is a treatable condition. Five cases had cataracts with correction of lesion after starting galactosemia regimen. Regarding the fact that cataracts can develop even in the first few weeks of life, early diagnosis of galactosemia and starting lifelong galactose-restricted diet can prevent hepatocellular insufficiency and other complications of the disease in future (15, 16). Also, a small percentage of infants was diagnosed with tyrosinemia, a treatable metabolic disease with heterogeneous symptoms including progressive liver failure, renal damage and pronounced coagulopathy. Today, expanded newborn screening, based on succinylacetone quantification has been very valuable in the early detection of hepatorenal tyrosinemia, providing the opportunity for rapid treatment of affected patients (17). This report highlighted the importance of neonatal screening for detecting treatable causes of IC.
The results of other evaluations such as Galactose-1-phosphate uridyl transferase assay, eye examination, succinylacetone quantification, and sweat test revealed metabolic disorders like galactosemia, tyrosinemia, and cystic fibrosis in some of cases. PFIC was diagnosed in an infant with positive family history. abnormality of glutamyl trans peptidase, pruritus which in follow up had suggestive histopathological features. Besides, liver cirrhosis was diagnosed in one infant due to biliary atresia. Reports of liver cirrhosis in Iranian children attribute it mainly to biliary atresia (18). This disorder requires more attention to come to an early diagnosis.
However, making a definite diagnosis of cholestasis in infants is not an easy task as there is no single and specific manifestation, laboratory test or imaging with 100% accuracy. In present study, jaundice was the main manifestation and there were significant differences in biliary atresia group who had more acholic stool, abnormal AST, ALT and total bilirubin (P < 0.05).
In the present study, clay-colored stools were significantly more common in infants with biliary atresia than in those with non-biliary atresia (P < 0.01). Our results are in accordance with authors, which reported acholic stool as a diagnostic sign (19, 20). This simple clinical finding is a very important sign in differentiating extrahepatic biliary atresia from neonatal hepatitis and could be used as important tool for evaluation of infantile cholestasis.
In our study, liver biopsy was able to differentiate BA from NH in 60.8% of cases. In a study by Yang et al, 69 infants underwent different modalities such as magnetic resonance cholangiography (MRCP), ultrasonography (US), hepatobiliary scintigraphy (HBS), and liver biopsy for differentiating idiopathic neonatal hepatitis from BA. Results of this research showed liver biopsy had the highest sensitivity in detecting BA at 100%, a specificity of 94.3% and an accuracy rate of 96.9% (21). Liver biopsy is considered to be a safe and an effective procedure in children with a low complication rate of 1.7% (22). In addition to its role in diagnosis, liver biopsy may also reveal histologic features of disease with significant prognostic value such as degree of fibrosis (6). However, the proper use of liver biopsy remains a central component of diagnostic evaluation of infant with cholestatic jaundice, as the differential diagnosis is the broadest of any age. Although it is an invasive method among the various tests, it was a safe procedure with no complications.
4.1. Conclusion
In conclusion, a variety of disorders can present with cholestasis during the neonatal period. In this study, almost 61% of all IC in our region were due to biliary atresia and idiopathic neonatal hepatitis and among other causes galactosemia was the more important one. As there is no single and specific clinical symptom as well as laboratory tests, imaging or pathology findings with 100% accuracy, a systemic approach is the key to reliably achieve a rapid diagnosis and confirm or rule out biliary atresia and other treatable disorders. Further research in this regard is highly recommended.