1. Background
Encouraging and maintaining breastfeeding is one of the most important ways of ensuring good health and promoting the optimal growth and development of infants. Therefore, it is considered one of the main components of primary health care, reducing maternal and neonatal problems, and improving cognitive development of infants (1, 2). Several problems such as a mother's inexperience in breastfeeding, lack of mother's adequate training and the use of supplements may lead to a reduction in milk intake and abnormal weight loss in the first week of neonatal life (3-7). An infant with dehydration and sodium retention will defend himself with reduction of urine volume and number of defecation to maintain body water and if this problem is not corrected, it can lead to neonatal hypernatremic dehydration (NHD) (8, 9). The incidence of hypernatremic dehydration in breastfeeding infants is 1 - 2% worldwide, whereas it is reported to be between 1-20% in developing countries (10).
Due to the fact that clinical symptoms of infants with NHD appear gradually, and signs of dehydration are often not visible in physical examination, clinicians or health care providers may not deduce the true extence of dehydration of the infant. Clinical symptoms of hypernatremic infants include jaundice, hyperthermia, lethargy, restlessness, mucosal dryness and weight loss of more than 10% (11). In the case of delayed treatment of these infants, serious complications such as seizure, cerebral edema, renal failure and even death may occur (12, 13). The follow-up of these infants has shown prolonged neurological complications in severe cases (1). Identification of predisposing factors for long-term complications has been less studied. One of the laboratory findings associated with NHD is thrombocytopenia. The incidence of thrombocytopenia in NHD and its role in the prognosis of these infants are unknown.
2. Objective
This study was conducted with the aim of evaluating the incidence and role of thrombocytopenia in the prognosis of NHD infants.
3. Methods
In a cohort study, we evaluated the platelet levels of term infants with hypernatremic dehydration and compared the prognosis of hypernatremic dehydration infants based on their platelet levels. This study was approved by the Ethics Committee of the Research Center of Mashhad University of Medical Sciences. Before entering the study, an informed consent was obtained from the parents of infants. This study consisted of 390 infants who were admitted to Ghaem Hospital in Mashhad during 2011 - 2017 by using available sampling methods. Infants with congenital disorders, infection and neonates whose parents were not able to cooperate in the follow-up, were excluded from the study.
Data collection tools in this research was a researcher-made questionnaire enquiring maternal characteristics: mode of delivery, breast problems and let-down reflex; neonatal characteristics: blood glucose, age, duration of hospitalization, birth weight, weight at the time of referral, Apgar score, first time of breastfeeding, frequency of breastfeeding, frequency of urination, frequency of defecation, first time of postpartum defecation, duration of feeding, sex, chief complaint and infant prognosis; laboratory characteristics of infants: serum levels of sodium, potassium, urea, creatinine, bilirubin, hematocrit, platelets, pH and glucose; and neonatal imaging characteristics: brain CT-scan, kidney ultrasound. All clinical and diagnostic examinations of infants were performed by a neonatologist.
At first, based on the serum level of sodium, the infants were divided into two groups: isonatremic (serum sodium level < 150 mEq/L) and hypernatremic (serum sodium level ≥ 150 mEq/L). In the next stage, the infants were divided into two groups: hypernatremic dehydration with normal platelet levels (platelets ≥ 150,000 /mcl) and hypernatremic dehydration with thrombocytopenia (platelets < 150,000/ mcl).
The infants with hypernatremic dehydration were followed up by using the Denver II Evolutionary Test Questionnaire at 6, 12, 18, 24, 30 and 36 months of age. The Denver II Evolutionary Screening Test is an international test to examine the process of children’s development from birth to the age of six years and evaluates children in 4 areas: 1) personal-social 2) subtle-adaptive movements 3) language (speaking) 4) rough movements. The titles in Denver II are carefully selected in terms of integrity of the norms in all subcategories and cultures.
When the infants had difficulty in each of the areas 1 to 4, they were regarded as developmentally delayed (14). After the assessment and follow-up of these children at 3 years of age they were divided into two groups of normal outcomes (normal development at 3 years of age) and abnormal outcomes (developmental delay or death). Then their blood platelet levels during neonatal admission for dehydration were compared.
3.1. Statistical Analysis
SPSS software version 20 was used to analyze the data. At first, results were described by using the tables and statistical charts and secondly, Chi-square and t-test were used to compare the two groups of infants with hypernatremic dehydration and normal platelet levels and infants with hypernatremic dehydration occurring with thrombocytopenia. ROC curve was used to determine the predictive factors of these children based on blood levels of sodium and platelet. P ≤ 0.05 was considered statistically significant.
4. Results
Among the 390 admitted infants, the mean age of infants was 7 days and the mean age of mothers was 26.5 years. The mean infant birth weight and weight at referral was 3174.56 and 3060.69 gr, respectively, and mean hospital stay was 3 days. Blood test analysis at admittance showed: sodium 148 mEq/l, potassium 5.5 mEq/l, urea 61 mg/dl, creatinine 1.02 mg/dl, total bilirubin 16.9 mg/dl, hematocrit 46%, platelet count 251312 /μl, blood glucose 121 mg/dl and pH 7.31 mEq/l.
Among the 390 admitted infants, 264 (68%) had serum sodium level < 150 (mEq/L) and 126 (32%) had serum sodium level ≥ 150 (mEq/L). Thrombocytopenia (platelets < 150,000 /μl) was observed in 41% of hypernatremic and 6% of isonatremic infants (P < 0.05) (Table 1).
Among the Hypernatremic neonates, the most common clinical symptoms at admission were jaundice in 30 (23.8 %), lethargy in 27 (21.4%) cases, feeding problems in 24 (19%), hyperthermia in 15 (11.9%), restlessness in 12 (9.5%), seizure in 6 (4.7%), and decreased consciousness in 4 (3.1%) infants, 8 (6.3%) cases were referred for routine examinations.
Mean daily weight loss, percentage of daily weight loss and percentage of total weight loss in hypernatremic group was 91g, 2.9%, 14.9%, and in isonatremia group 31g, 0.6% and 1.7%, respectively.
In this study, infants with NHD were divided into two groups: those with low platelet (n = 52) and normal platelet (n = 74) counts (Table 1). The mean age at admission was 5.63 days in the normal platelet group and 9.43 days in the thrombocytopenia group. The percentage of total weight loss was 18% in the group of hypernatremic infants with thrombocytopenia and 12% in the group of normal platelet infants. Serum urea and creatinine in infants with normal platelets were 95.93 mg/dl and 1.26 mg/dl, while in the low platelets group, these values were 201.69 mg/dl and 3.61 mg/dl, respectively. The mean blood glucose in the infants with normal platelet was 83.93 mg/dl, while 151.7 mg/dl were seen in low platelet group.
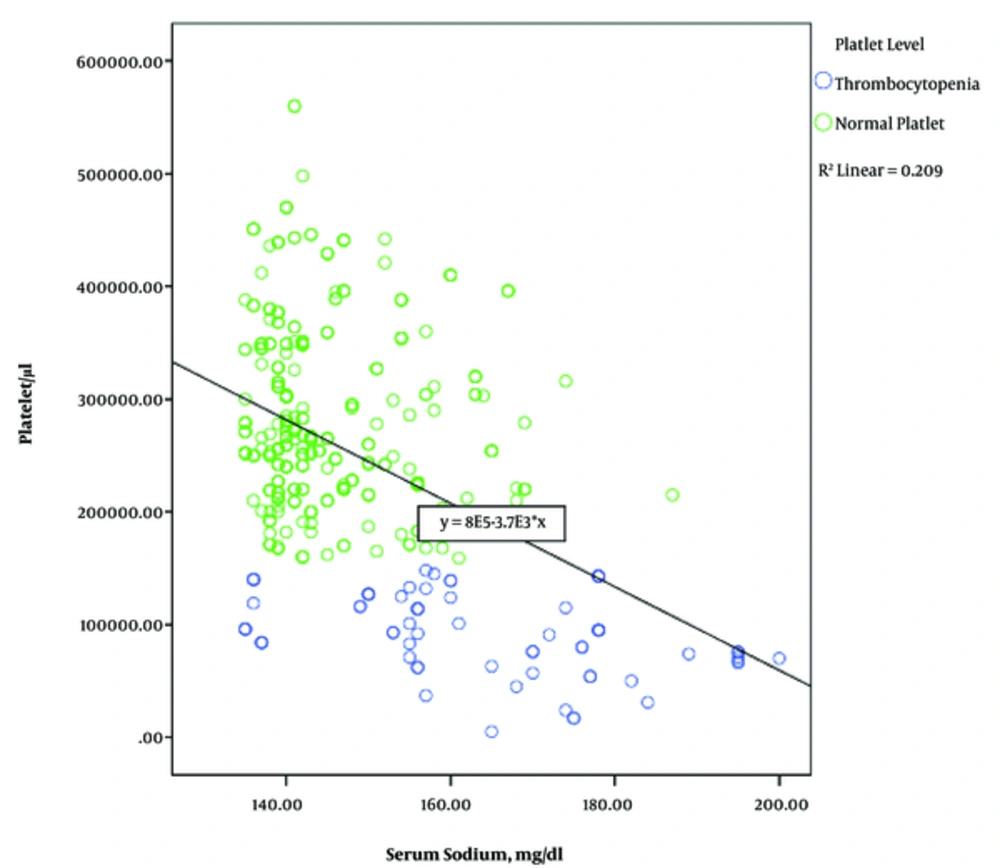
The mean of sodium levels in infants with normal platelets was 159.94 mEq/l, while in the low platelets group, it was 170.11 mEq/l. The Pearson Correlation Coefficient shows that with increasing serum sodium levels, serum platelet levels decrease (Pearson correlation = 0.435) (Figure 1).
No significant difference was found between these two groups in terms of hospitalization duration, blood pH, hematocrit, potassium and bilirubin (Table 2). Independent risk factors were evaluated between thrombocytopenic and normal platelet patients and a significant difference was found between the two groups in terms of sex, sodium level and prognosis (P < 0.05) (Table 3).
| Factor | Normal Platelet | Thrombocytopenia | P Value |
|---|---|---|---|
| Age, day | 5.63 ± 0.43 | 9.43 ± 0.67 | < 0.0001 |
| Birth weight, g | 3198.24 ± 47.71 | 3146.53 ± 68.07 | 0.522 |
| New weight, g | 2785.27 ± 44.83 | 2586.53 ± 68.06 | 0.012 |
| Hospitalization, day | 4.61 ± 0.85 | 7.00 ± 2.14 | 0.218 |
| Blood Sugar, mg/dL | 83.93 ± 18.78 | 151.70 ± 21.18 | 0.021 |
| Urea, mg/dL | 95.93 ± 10.84 | 201.69 ± 18.50 | 0.005 |
| Creatinine, mg/dL | 1.26 ± 0.13 | 3.61 ± 0.36 | < 0.0001 |
| K, mEq/L | 5.32 ± 0.12 | 6.54 ± 0.87 | 0.171 |
| Na, mEq/L | 159.94 ± 1.00 | 170.11 ± 1.88 | 0.0004 |
| pH, mEq/L | 7.35 ± 0.06 | 7.22 ± 0.10 | 0.07 |
| Bilirubin, mg/d | 15.65 ± 0.53 | 15.97± 1.54 | 0.810 |
| Hematocrit, % | 49.91 ± 0.93 | 49.67 ± 1.75 | 0.904 |
Hematological and Biochemical Characteristics in the Patient with and Without Thrombocytopeniaa
| Factor | Normal Platelet | Thrombocytopenia | P Value |
|---|---|---|---|
| Gender | 0.0005 | ||
| Male | 53 | 21 | |
| Female | 21 | 31 | |
| Mode of delivery | 0.50 | ||
| Normal Vaginal Delivery (NVD) | 53 | 28 | |
| Caesarean Section (CS) | 21 | 24 | |
| Prognosis | < 0.0001 | ||
| Developmental delay | 5 | 11 | |
| Normal | 69 | 39 |
Independent Risk Factors for Thrombocytopenia Between Two Groups of NHD During Admission
In thrombocytopenic group, seizure and cerebral hemorrhage were seen in 7 (13.4%) each and brain edema in 2 (3.8 %) cases. In the infants of normal platelet group, brain edema and periorbital edema were seen in 3 (4%) patients each, brain hemorrhage in 2 (2.7%) and seizure in 1 (1.3%). The final prognosis of patients in thrombocytopenic group was significantly poorer than in normal platelet group (P < 0.001). Among 52 hypernatremic patients with low platelet counts, 11 (21.15%) infants suffered from developmental delays at 2 years of age and 4 (7.7%) infants died during hospitalization, whereas among 74 hypernatremic infants with normal platelet levels, 5 (3.3%) infants experienced developmental delays.
5. Discussion
Based on the results of this study, the occurrence of thrombocytopenia in NHD infants was found to be 7 times higher than in other infants. NHD infants who had thrombocytopenia were referred later to the hospital, had greater weight loss, higher serum urea, creatinine, sugar and sodium levels with more cerebrovascular complications and higher mortality.
In previous studies, no information about confirming or rejecting the afore-mentioned condition has been reported. No results have yet been reported about the presence of thrombocytopenia in patients with hypernatremic dehydration. This study is the first study which has investigated the incidence of thrombocytopenia in NHD infants; its risk factors and its impact on the prognosis of these infants. This finding was reported only in one case report. In the study of Omar Suliman, a twelve-day-old baby in poor general condition, lethargy and evidence of severe dehydration was admitted. The patient did not have cyanosis or jaundice, but had a 33% weight loss compared to birth weight. Laboratory assessment at admission showed severe hypernatremia (serum sodium level of 191 mmol/L), severe hyperglycemia (blood glucose 54 mmol/L), prerenal azotemia and thrombocytopenia. Due to poor general condition and the presence of thrombocytopenia, sepsis was suspected, intravenous antibiotics were given until the cultures were negative (15).
The reason for high incidence of thrombocytopenia in NHD is unclear, although it is possible that severe hypernatremia has an inhibitory effect on the bone marrow's platelet production, or it may be due to excessive peripheral consumption of platelets.
Mean age of our pstients at admission was 7 days, while the infants with hypernatremic dehydration with thrombocytopenia were referred about 4 days later in comparison to NHD cases with normal platelets. Generally, infants with hypernatremic dehydration were usually referred to hospital from 3 to 20 days of age (4, 9, 16-19). It seems that delayed referral can cause dehydration and hyperthermia and increase the risk of thrombocytopenia, mechanism of which is unknown. Weighing and examining of neonatal infants on 3 - 5 days of life assists in early detection and prevention of these complications (20).
In our patients, the percentage of weight loss was 14.9% in hypernatremic group and 1.7% in isonatremic infants. This rate was 18% in group of hypernatremia with thrombocytopenia and 12% in NHD infants with normal platelets. Naturally, infants lose about 7% of their weight during the first five days of life and often reach birth weight at seventh day of age (21, 22). An important and obvious sign of hypernatremic dehydration is significant weight loss. The relationship between weight loss and hypernatremic dehydration has been reported in several studies (23-27), though in our study the cause of higher weight loss (about 1.5 times) in thrombocytopenic NHD infants compared to ones with normal platelet levels is indistinct. Perhaps, delayed referral was one of the reasons leading to weight loss and hypernatremia and then thrombocytopenia.
In this study, thrombocytopenic NHD patients had higher urea and creatinine levels than normal platelets group, and renal insufficiency was more severe in these infants (P < 0.05). Failure in adequate breastfeeding of the infant can lead to various complications, including dehydration, uremia, rise of creatinine and hypernatremia. In the case of reduced intake of breast milk, neonate's kidneys attempt to reabsorb urine minerals and maintain fluid as a defensive mechanism. If dehydration is severe or treatment is delayed, hypernatremia and prerenal azotemia may occur. Also, the insensible water loss from the skin and lungs is continued due to lack of adequate maturity of the infant's skin and can cause these problems (24-26).
In our NHD thrombocytopenic infants, blood glucose was twice that of the infants with normal platelet levels. It has been mentioned that hypernatremia can be associated with hyperglycemia, but its mechanism is still unknown. Significant stress response of body to hypovolemia, in addition to impaired tissue sensitivity to insulin may be its cause. It is not necessary to administer insulin in treating hyperglycemia with hypernatremic dehydration, because with rehydration and correction of hypernatremia, hyperglycemia will also be amended (28). Tarkan et al. reported 3 infants with severe hypernatremia, all having acute renal failure, hyperglycemia and needing dialysis. It was reported that in hypernatremic dehydration, hyperglycemia increases the hypertonicity of body fluids and thus increases mortality (29). In our study, the higher occurrence of hyperglycemia in NHD infants who had thrombocytopenia may be due to higher sodium level in this group and it could be a sign of more severe hypernatremic dehydration.
In this study, seizure in NHD thrombocytopenic infants occurred significantly higher than in infants with normal platelet levels (P < 0.001). Seizure attacks may result from increased sodium level in brain cells and augmented intracellular osmolality during water loss (20). Increased blood osmolality following hypernatremia may cause brain damage associated with bleeding and seizure. On the other hand, seizure often occurs during treatment of hypernatremia when sodium is returning to normal level, though it is also common even without obvious pathological lesions (30-33). However, the cause of higher seizure occurrence in thrombocytopenic NHD infants in this study is indeterminate. It is reasonable that severe complications occur more with higher sodium levels.
Based on the results of this study, thrombocytopenia in NHD increases the risk of death by about eight times (P < 0.001). The developmental delay was also significantly higher in these patients. This could be due to greater severity of the disease in this group. The study of Ergenekon et al. which consisted of 28 NHD infants showed that among 15 patients who participated in long-term follow-up, severe developmental delay was recorded in 2 (13%) cases (34).
5.1. Conclusion
The results of our study for the first time showed strong relationship between hypernatremia and thrombocytopenia in infants with dehydration. NHD increased the possibility of association with thrombocytopenia by about 7-fold. NHD thrombocytopenic infants were referred later and had greater weight loss. Urea, creatinine, glucose and sodium were higher in this group, and more brain complications and mortality have been recorded in these infants.
Based on the results of this study, it seems that in addition to routine measures for diagnosis of NHD, special attention to serum platelet levels may be helpful in controlling acute problems in these infants and is also effective in predicting long-term complications; however, more studies in this area seem to be necessary.
5.2. Limitations
One of the limitations of this study is not using more accurate paraclinical evaluations such as brain MRI and the other, is use of Denver Testing alone for assessing developmental delay.