1. Background
Cystic fibrosis is the most common deadly genetic disease among white individuals. It is an autosomal recessive hereditary disease and is the outcome of mutations in cystic fibrosis Trans membrane regulator (CFTR). A total of 2000 different mutations have been identified in this gene. Distribution and type of these mutations differ in different populations (1-5). Pulmonary complications are the leading cause of morbidity and mortality in this disease. Involvement of the upper respiratory tract and pathological changes in the nose and paranasal sinuses are common in patients with cystic fibrosis. Many patients do not report Sino nasal symptoms. Involvement of the upper respiratory tract occurs as chronic rhino sinusitis and nasal polyps that can affect the quality of life in these patients (6-9). Nasal polyps are the most common finding on physical examination. Almost all patients with cystic fibrosis have radiological and sinus changes in their nose, however, these changes are not associated with disease severity (10, 11). Nasal and paranasal sinus opacity, medialization of the lateral nasal wall, demineralization of uncinate excrescence, hypoplasia, and agenesis of frontal and sphenoid sinuses are among the most common CT findings in these patients (12). With respect to cystic fibrosis, few studies have been carried out in Iran. Few studies examining the genetics of this disease have shown that most Iranian patients have a range of heterogeneous mutations, which are mainly heterozygotes consisting of a severe mutation and one or two mild mutations; therefore, Iranian patients often have a milder phenotype.
Despite numerous studies in other countries, there is still no study on the sinonasal manifestations among these patients in our country (13-16).
2. Objectives
We aimed to identify sinonasal manifestations in Iranian children with cystic fibrosis.
3. Methods
In this study, 47 children diagnosed with cystic fibrosis, based on standardized tests, were enrolled consecutively during one-year period. The study protocol was fully explained to the parents and their written informed consent was obtained. Primarily questionnaires regarding common sinonasal complaints such as the existence of nasal discharge, nasal congestion, facial pain, headache, postnasal drip, open mouth breathing, night snoring, and bad breath in the case of older children and their compliance were completed by asking the parents.
Children underwent thorough examination of the mouth, throat, and nose. After local anesthesia of the nasal cavity with lidocaine 1%, children underwent nasal endoscopy using 0 degree rigid endoscopes and the nasal cavity was examined for the presence or absence of polyps. If the patient had already undergone nasal and paranasal sinus CT scans for any reason, the related scans were assessed. In addition, the findings related to the presence or absence of opacity in the nasal cavity and sinuses, presence of medialized lateral nasal wall, hypoplasia of the sinuses, nasal septal deviation, and concha bullosa were evaluated. In case of absence of CT scans in the past, CT scans were performed and examined. Patients were excluded if there was diagnostic uncertainty regarding cystic fibrosis or nasal and sinus disease that affects symptoms of patients such as acute rhinosinusitis, intranasal masses, or common cold. All patients were visited by Allergy Immunology Department co-workers of our survey and if we found positive signs or symptoms we excluded them from the study.
Data were analyzed using SPSS software, version 20. Descriptive statistics (mean ± standard deviation, frequency and percentage), Fisher’s exact test for qualitative variables, Mann-Whitney U test for the quantitative variables, and Kolmogorov-Smirnov test for normal distribution of data were used as indicated. P < 0.05 was considered statistically significant.
4. Results
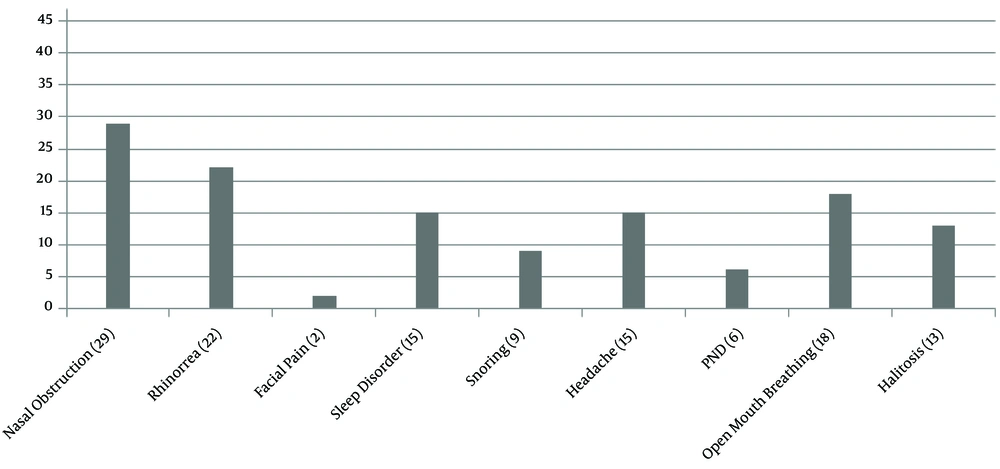
The age of the patients was 9.38 ± 4.2. The children had a weight of 25.3 ± 14.05 kg and heights of 118.1 ± 24.5 cm. A total of 28 (59.6%) patients were male. The frequency of common nasal and paranasal sinus symptoms correlating with cystic fibrosis is shown in (Figure 1).
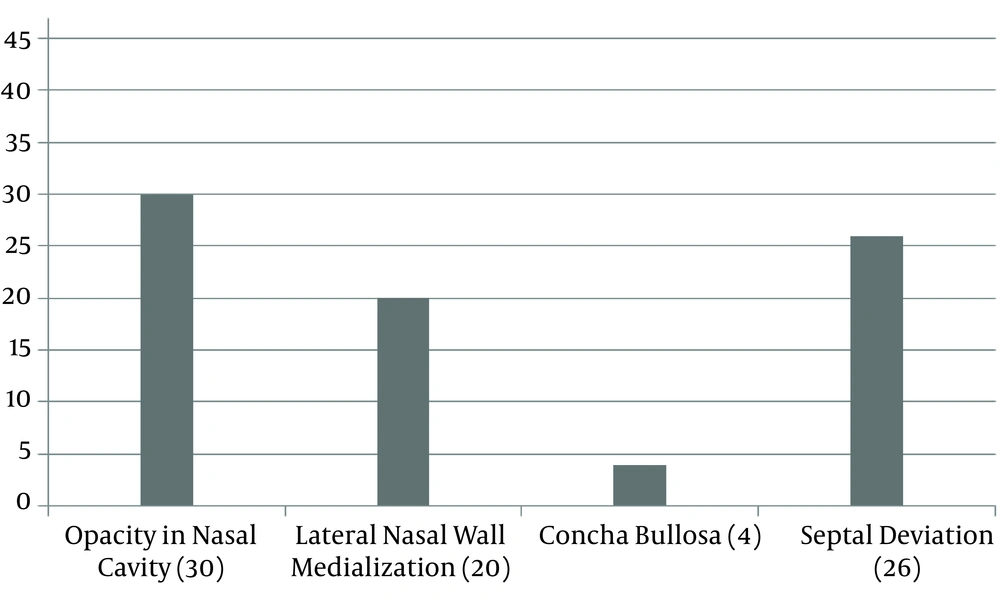
A total of 44.7% of patients had a medical history of at least one time use of intranasal corticosteroid, however, none of the patients had a history of endoscopic nasal surgery. A total of 24 (51.1%) patients had a hypoplasia of at least one paranasal sinus, as follows: two (4.3%) patients with hypoplasia of the ethmoid bone, nine (19.1%) with hypoplasia of the sphenoid bone, one (2.1%) with hypoplasia of the maxilla, and 23 (48.9%) with hypoplasia of the frontal sinus. Nasal CT scan results are shown in (Figures 2 and 3).
In 34 (72.3%) patients, at least one sinus was opacified. A total of 53.2% of the opacifications were found in the ethmoid bone, 31.9% in the sphenoid bone, 74.5% in the maxilla, and 14.9% in the frontal sinus. Dental caries were seen in 61.7% of patients. Premolar teeth were the most common caries teeth (48.9%). A total of 61.7% of patients had purulent rhinorrhea, of which 36.2% had post nasal discharge and 31.9% had nasal polyp (one unilateral, 13 bilateral). The mean of tonsillar grade was two. There was a significant correlation between age and nasal polyps. All patients with polyps also had severe clinical phenotype so F508∆ mutation, especially the homozygote type, was more common in patients with polyps. All patients with polyps also had severe clinical phenotype. Three patients had a severe disease state. We don’t have any bacteriology information on our patients due to precise and acceptable bacterial information of sinus need Trans sinus osteomeatal complex aspiration under general anesthesia, therefore, we didn’t do it. Lower airway samples (cough swabs or sputum) were taken from 37 children with cystic fibrosis (median age 10 years). Samples cultured eight Pseudomonas aeruginosa (PA), eight Staphylococcus aureus (SA), and 12 other bacterial pathogens.
We found no significant correlation between polyps and nasal congestion, rhinorrhea, facial pain, snoring, open mouth breathing, and halitosis (P = 0.05) (Figure 1).
5. Discussion
Disease of the nose and paranasal sinuses is almost present. In all patients with cystic fibrosis, the complaints are usually not reported by patients, due to the fact that these symptoms are usually present at birth and children are adapted to them. Many of the previous studies referred to nasal congestion as the most common symptom of the disease. In the present study, the most common complaint in children was nasal congestion (61.7%). In addition, there was no relationship between symptoms and age, sex, height, and weight that was consistent with the most recent studies (9, 17, 18). In our study, 31.9% of nasal polyps were diagnosed using rigid endoscope examination. In previous studies, this diagnosis rate has been reported to be very variable. In a literature review by Carvalho and colleagues, the incidence of polyposis in patients with cystic fibrosis varied from 6% to 67% (17) . In another study, 44% of patients had nasal polyps (19). It should be noted that these studies employed different methods for diagnosing polyps. Some of them just used anterior rhinoscopy, while most studies used an endoscopy with different methods. In most studies, similar to our study, rigid endoscope was used in outpatients (20, 21). In a study, rigid endoscope was done in the operating room under general anesthesia, while Freitas and colleagues used flexible endoscopy in outpatients (20, 21).
By reviewing previous studies, the diagnosis of rhino sinusitis and nasal polyps in patients with cystic fibrosis was different (history, CT scan, and nasal endoscopy). Almost all patients have some degree of radiological changes and evidence of disease of the nasal mucosa and sinuses. Various studies have offered different figures of these findings. However, the over diagnosis of nasal polyposis, based on radiological findings, has been common in most studies (11, 22). In our study, the most common sinus with opacification was the maxillary sinus with 5.74% involvement, followed by ethmoid involvement (54.2%). This was consistent with most previous findings. In a study on 34 patients with cystic fibrosis at the age of six, Boari and co-workers evaluated the results of the questionnaires, nasal endoscopy, and Sino nasal CT scans in the diagnosis of chronic rhino sinusitis. According to this study, the most commonly involved sinuses were the maxillary sinus (91.9%) and the anterior ethmoid (83.9%). According to this study, the three different methods had different diagnosis power for detecting rhino sinusitis. This diagnosis power was more in CT scan and less in questionnaire-based reviews (23). In our study, the least amount of involvement was observed in the frontal sinus, which is justified considering that the present study was conducted on the pediatric population and considering the evolution of the frontal sinus at older ages. We also found some degrees of hypoplasia in at least one of the sinuses (51.5%) and the frontal sinus accounted for the highest percentage (48.9%). Frontal sinus agenesis is one of the notable features among patients with cystic fibrosis and chronic rhino sinusitis (9, 22, 24) . In this study, there was no statistical relationship between the presence of polyps with the examination, age, and symptoms, as well as examination findings, such as rhinorrhea, and sinonasal CT findings. Accordingly, it can be stated that the patients’ history, CT scans, and physical examinations (rhinorrhea in anterior rhinoscopy) did not allow accurate diagnosis of chronic rhinosinusitis with nasal polyps in children with cystic fibrosis and nasal endoscopy is the most reliable method for the diagnosis of nasal polyps.
5.1. Conclusions
Sinonasal manifestations are common in children with cystic fibrosis. These patients should undergo periodic examinations by an otolaryngologist. Nasal endoscopy is the best method for the diagnosis of rhinosinusitis with nasal polyps in these patients.