Dear Editor,
The incidence of thromboembolic events in the pediatric age group is higher in neonates (1).
In a German study the incidence of thromboembolc events in the neonatal period was 5.1 per 100000 births (2) and 2.4 per 1000 NICU admissions with 45% to 55% of these events occurring in preterm infants (3). Risk factors for thromboembolic events are infection, polycythemia, gestational diabetes mellitus of mothers, perinatal asphyxia and inherited thrombophilia such as factor 5 Leiden and deficiency of protein C and S, dehydration and shock, congenital heart disease, umbilical venous and arterial catheters (1, 4). Thromboembolic events in the neonatal period often require urgent intervention to restore perfusion and to prevent morbidity and even mortality. The most common clinical findings are irritability, absent peripheral pulses, pallor or duskiness of extremities, coldness of the lower extremities and difference between the upper and lower extremities' tensions. Diagnosis of arterial thrombosis is confirmed by ultrasonography. Therapeutic options include anticoagulation, fibrinolytic therapy, surgical thrombectomy or combination of these therapies according to location of thrombosis and clinical findings.
Our case was a male neonate born to a 30 years old primigravida by cesarean section due to oligohydramnios and preeclampsia at 36 weeks of gestational age with Apgar score of 9/10. His birth weight with 2450 gram was appropriate for gestational age. The neonate was hospitalized for phototherapy. In the third day of life he was discharged by parental consent. At the fifth day of birth the neonate was readmitted to NICU with poor feeding and severe dehydration with more than 30% weight deficit and oliguria, hypotonia, icterus, severe prerenal azotemia. Na 178, K 5, BUN 70, Cr 1; Urinalysis: specific gravity 1030, pH 5, blood 1+, protein 2+; U/C positive for Ecoli. CBC: white cells 7600, neutrophils 64%, lymphocytes 29%. Thrombocytopenia with normal other indices. Hypernatremia and azotemia was corrected and jaundice disappeared but in the third day coldness and cyanosis in extremities was noted. Concurrently platelets decreased and PT and PTT were prolonged. Prothrombotic conditions such as presence of factor 5 Leiden; deficiency of protein C, S and antithrombin, lipoprotein a and abnormalities or deficiencies of homocysteine; fibrin degradation products (FDP), D-dimers, fibrinogen were noticeable. Lupus anticoagulants and antiphospholipid antibodies as well as ANA which are known risk factors for thrombosis were checked in the patient and his mother. Initially protein C was very low in the patient but after treatment with LMWH, it increased and became normal which indicated that inherited protein C deficiency could be ruled out.
FDP and D-dimer in the patient were high whereas fibrinogen, AST and ALT were in normal range. ANA, Antiphospholipid antibodies, Anticardiolipin antibodies was negative and Protein C, S, and homocysteine was in normal range when screening for a possible inherited thrombophilia disorder the mother. His vital signs were normal but the cyanosis of his extremities was progressive. In addition his echocardiography showed a moderate ASD2 (Atrial septal defect) and mild pulmonary hypertension with ejection fraction of 60%. There was no organomegaly. Many inherited thrombophilia illnesses like prothrombotic polymorphisms can also increase risk of neonatal arterial thrombosis (5). Our patient had no prothrombotic polymorphism.
In conclusion it can be accepted that dehydration and urosepsis as predisposing factors caused the arterial thromboembolism in our patient.
| Parameters | Patient | Normal Range |
|---|---|---|
| Pr C first test | 17.9 | 70 - 140 IU/dL |
| Pr C second test | 70 | 70 - 140 IU/dL |
| Pr S | 61.4 | 60 - 150 IU/dL |
| FDP | > 10 | < 10 mg/dL |
| D-dimer | 2142 | < 500 ng/mL |
| Fibrinogen | 210 | 150 - 400 mg/dL |
| Anticardiolipin antibodies IgG | 0.3 | < 10 u/mL |
| Anticardiolipin antibodies IgM | 1.3 | < 7 u/mL |
| Antiphospholipid antibodies IgG | 1 | < 10 u/mL |
| Antiphospholipid antibodies IgM | 1.5 | < 7 u/mL |
| Homocysteine | 7 | < 5 - 15 µmol/L |
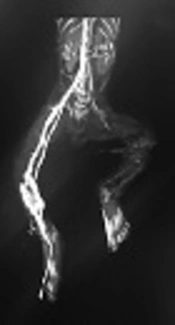
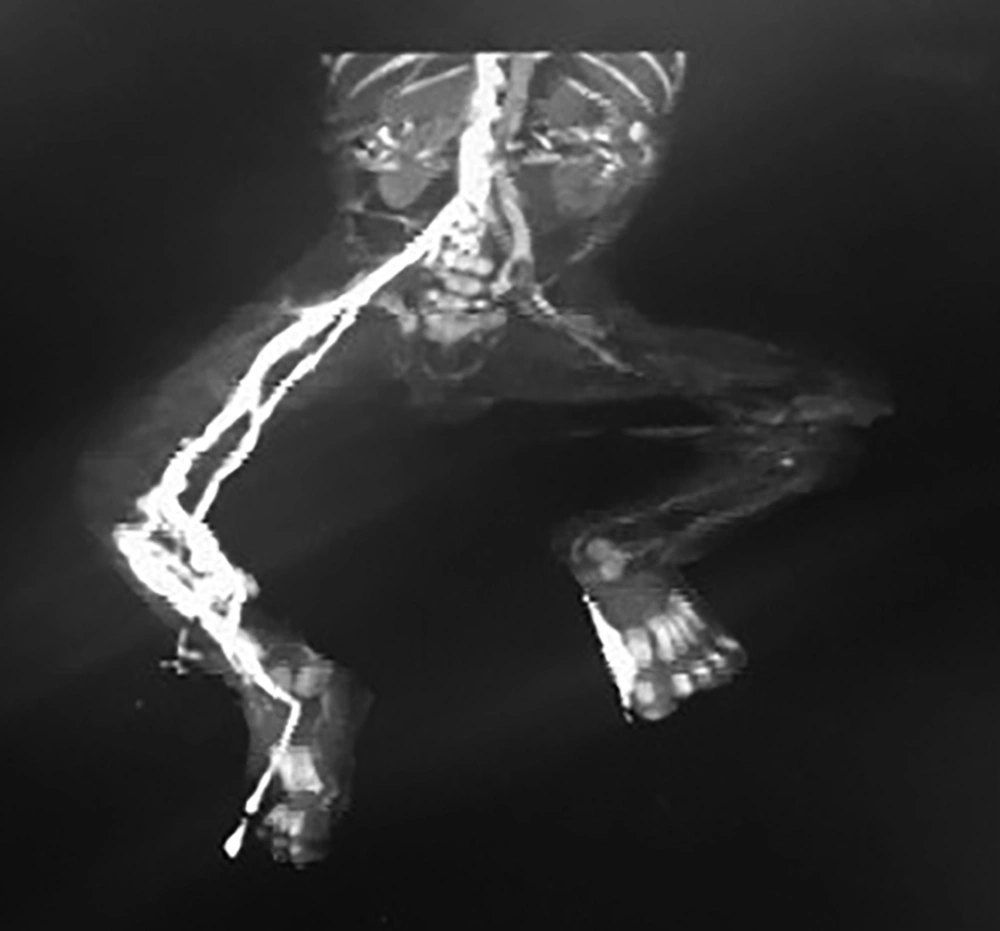
Brain sonography and brain MRI were normal. Kidney sonography revealed mild hydronephrosis of the right kidney, kidney size: right 42 × 19 cm, left 40 × 17 cm. In Doppler ultrasonography normal blood flow in renal veins, no flow was detected from the left popliteal artery to distal extremity. The veins had normal flow. In angiography; partial thrombosis was seen in abdominal aorta from infra renal to bifurcation of aorta which is very rare in neonates.
After confirming the diagnosis, unfractionated heparin was administered as intravenous infusion which was switched after two days to Enoxaparin, a kind of LMWH (low molecular weight heparin). Because of difficult sampling for monitoring of UFH and difficulty in maintaining target levels and because the patient did not respond to UFH, the dose of enoxaparin was titrated with anti Xa factor measurement. The initial dose of Enoxaparin of 1.5 mg/kg per dose was injected subcutaneously every 12 hours which was monitored to a target range of 0.5 to 1.0 units/mL in a blood sample taken 4 to 6 hours after subcutaneous injection. Then a higher dose (2.2 mg/kg/dose) was administered subcutaneously every 12 hours in order to attain the minimum anti-factor Xa activity of 0.5 units/mL for therapeutic anticoagulation, which is higher than its upper limit that indicated in literature.
During treatment we used intermittent Doppler ultrasonography to estimate the progression of thrombosis. Ultrasound imaging was obtained before and during the antithrombotic therapy to confirm the absence of intracranial hemorrhage (ICH).
The urosepsis was treated with widespread antibiotics. FFP was transfused only three times for correction of acquired protein C deficiency. Skin injury treated with TNG ointment and with alcohol wound dressing. Finally all symptoms and signs disappeared. We continued anticoagulation therapy up to 6 months and followed him. He grew well and had an appropriate neurodevelopment with no recurrence of thromboembolism. After one year Doppler ultrasonography was repeated and no thrombosis was revealed.
In spite of limited evidence on the safety of anticoagulant treatment in neonates, LMWH (specifically enoxaparin) has become the anticoagulant of choice for neonates. Antithrombotic treatment must occur in a NICU equipped with adequate laboratory, and access to neonatal hematology, transfusion, medicine, and pediatric surgical support.
In summary, thromboembolic events in the neonatal period require urgent intervention. LMWHs are the best choice for treatment of neonatal arterial thromboembolism. The dose of enoxaparin must be titrated with anti Xa factor measurement.