1. Background
Peripheral neuroblastic tumors (PNTs), a family of tumors of the sympathetic nervous system, account for 7% to 10% of all tumors in children (1-3). According to the different cells of origin, neuroblastic cells and Schwannian cells, PNTs were classified into three histologic types as follows: neuroblastoma, ganglioneuroblastoma (nodular and intermixed) and ganglioneuroma (4). Only neuroblastomas and nodular ganglioneuroblastomas are considered malignant, as they easily lead to death without treatment. On the contrary, intermixed ganglioneuroblastomas and ganglioneuromas are considered to be benign and curable by surgery alone (5).
PNTs may develop at any site of the sympathetic nervous system tissue. Most primary PNTs occur in the abdomen, frequently in the adrenal medulla; others develop at various sites of the paraspinal sympathetic ganglia, including the neck, chest, and pelvis (6, 7). Tumors originating from different sites may have different clinical characteristics and outcomes. If PNTs in children are correctly diagnosed and properly treated at an early stage, most are curable; if they are improperly managed, this may even lead to death, especially in the case of neuroblastomas. Pediatric PNTs seem to have a consistent clinical and biological course.
In our review of the medical literature, we found only small case series regarding the symptoms and presentations of PNTs in children, most of which only focused on abdominal or extra-abdominal PNTs (3, 8-11). To better understand the clinical characteristics and management of pediatric PNTs, we reviewed a series of 43 patients with PNTs treated at our institute over the last 10 years.
2. Methods
From January 2007 to December 2016, a total of 43 patients who were newly diagnosed with PNTs at our hospital were retrospectively reviewed. All the clinical details and related data were collected from the department’s data base. The inclusion criteria were as follows: all patients first visited our hospital and received a pathologic diagnosis of PNT based upon surgical specimens or biopsy specimens during the specified time period. The study protocol involving human materials was approved by the institutional ethics committee of The Second Affiliated Hospital and Yuying Children's Hospital of Wenzhou Medical University.
3. Results
From 2007 to 2016, 43 pediatric patients were diagnosed with PNTs. Table 1 shows the characteristics of the patients with PNTs. The median age of patients was 1.2 years old (ranged from 14 days to 12 years). PNTs were more frequent in patients < 1 year old than in patients > 5 years old, in which PNTs were rare. The patients included 23 male (53.5%) and 20 female (46.5%). There was no significant difference in the overall incidence of PNTs between the sexes. The most common primary lesion was in the abdomen, occurring in 27 (62.8%) patients, including in the retroperitoneum in 16 (37.2%) patients, and in the adrenal gland in 11 (25.6%) patients. The second most common primary site was the thorax, occurring in 13 (30.2%) patients. Other rare sites included the neck (n = 1), vertebral canal (n = 1), orbit (n = 1), and pelvis (n = 1). The size of the tumors ranged from 3 × 2 cm to 17 × 13 cm. The diameters of most abdominal PNTs were greater than 5 cm, especially in the retroperitoneum where the maximum tumor diameter was 17 cm.
| Abdomen | Thorax | Other | Total | |
|---|---|---|---|---|
| Number | 26 | 23 | 4 | 43 |
| Gender | ||||
| Male | 18 | 3 | 2 | 23 |
| Female | 8 | 10 | 2 | 20 |
| Age, y | ||||
| < 1 | 13 | 7 | 3 | 23 |
| 1 - 5 | 12 | 5 | 1 | 18 |
| > 5 | 1 | 1 | 0 | 2 |
| Size, cm | ||||
| < 5 | 11 | 10 | 3 | 24 |
| > 5 | 15 | 3 | 1 | 19 |
| Symptoms | ||||
| Routine examination | 13 | 3 | 0 | 16 |
| Abdominal pain / distention | 6 | 1 | 0 | 7 |
| Cough | 0 | 7 | 0 | 7 |
| Fever | 5 | 1 | 0 | 6 |
| Palpable mass | 1 | 1 | 2 | 4 |
| Other | 1 | 0 | 2 | 3 |
| Laboratory studies | ||||
| VMA | 15 | 3 | 0 | 18 |
| NSE | 10 | 2 | 0 | 12 |
| LDH | 2 | 0 | 0 | 2 |
| Ferritin | 1 | 0 | 0 | 1 |
As shown in Table 1 16 patients (37.2%), whose tumors were identified in routine examinations, exhibited no clinical signs or presentation. Among these patients, nine patients were identified during pregnancy. The remaining 27 patients had obvious symptoms and signs. The main symptoms of abdominal PNTs were abdominal pain or abdominal distension, which was observed in six patients. The duration of symptoms ranged from one week to four months, with increasing severity. The main symptom of thoracic PNTs was coughing, which was seen in 7 patients. Other common symptoms included fever, occurring in six patients, and a palpable mass, occurring in four patients. Rare symptoms included weakness in a limb (n = 1), diarrhea (n = 1), and hoarseness (n = 1).
Radiological studies, such as ultrasonography (US), enhanced abdominal computed tomography (CT) scan, or magnetic resonance imaging (MRI) showed a mass in all of the patients. The vanillylmandelic acid (VMA) level in the urine over 24 hours was a routine diagnostic evaluation. Of 30 patients with neuroblastoma, 18 (60%) patients tested positive, including an abnormal VMA level over 24 hours (ranging from 55 µmol/24 hours to 549 µmol/24 hours). However, of the 11 patients with ganglioneuroblastoma, only three patients tested positive for VMA (ranging from 80 µmol/24 hours to 150 µmol/24 hours). Serum neuron-specific enolase (NSE), lactic dehydrogenase (LDH), and ferritin were detected in 26, 10, and eight patients, respectively. NSE was elevated in nine neuroblastoma cases, two ganglioneuroblastoma cases, and one ganglioneuroma case (ranging from 26 ng/mL to > 370 ng/mL). Only two patients with neuroblastomas had a high level of LDH and one patient with a neuroblastoma had a high level of ferritin.
All of the patients presented pathological findings. As shown in Table 2 the tumors of 28 patients were resected and 15 tumors were biopsied. Neuroblastoma was the most common type of PNT and was reported in 30 (69.8%) patients. Ganglioneuroblastoma was the second most common type of PNT, and it was diagnosed in 11 (25.6%) patients. Among these, nine were intermixed ganglioneuroblastomas and two were nodular ganglioneuroblastomas. Only two (4.6%) patients were diagnosed with ganglioneuromas. According to the Shimada classification (5), 17 tumors had unfavorable histology (UFH), and 26 tumors had favorable histology (FH). According to the international staging system (INSS) (12), 12 cases were stage I, six cases were stage II, three cases were stage III, 18 cases were stage IV, and four cases were stage IVs.
| Abdomen | Thorax | Other | Total | |
|---|---|---|---|---|
| Number | 26 | 23 | 4 | 43 |
| Surgery | ||||
| Biopsy | 12 | 2 | 1 | 15 |
| Resect | 14 | 11 | 3 | 28 |
| Pathology | ||||
| Neuroblastoma | 20 | 8 | 2 | 30 |
| Ganglioneuroblastoma | 5 | 5 | 1 | 11 |
| Ganglioneuroma | 1 | 0 | 1 | 2 |
| Shimada classification | ||||
| FH | 13 | 9 | 4 | 26 |
| UFH | 13 | 4 | 0 | 17 |
| Stage | ||||
| I | 4 | 6 | 2 | 12 |
| II | 2 | 4 | 0 | 6 |
| III | 2 | 0 | 1 | 3 |
| IV | 15 | 2 | 1 | 18 |
| IVs | 3 | 1 | 0 | 4 |
Certain patients with malignant tumors were treated with chemotherapy, which consisted of different combinations of vincristine, cisplatin, etoposide, cyclophosphamide, and carboplatin (13-15). Additionally, some patients gave up treatment after the diagnosis. Patients were followed up for one to three years after diagnosis, and eight patients were lost during that time frame. The two year survival rate was based on the 43 patients diagnosed between 2007 and 2016. As shown in Table 3 the overall two year survival rate was 62.9%. The two year survival rate for neuroblastoma, ganglioneuroblastoma, and ganglioneuroma were 47.8%, 90%, and 100%, respectively. The two year survival rate for the FH group was 90.5%, whereas the two year survival rate in the UFH group was 21.4%. The two year survival rate, according to tumor stage, was as follows: stage I 88.9%, stage II 100%, stage III 100%, stage IV 25.7%, and stage IVs 25%. The 2-year survival rate, according to the primary tumor sites was as follows: abdomen 59.1%, thorax 66.7%, and others 75%.
| Total | Follow-Up | Survival | Survival Rate, % | |
|---|---|---|---|---|
| Number | 43 | 35 | 22 | 62.9 |
| Pathology | ||||
| Neuroblastoma | 30 | 23 | 11 | 47.8 |
| Ganglioneuroblastoma | 11 | 10 | 9 | 90 |
| Ganglioneuroma | 2 | 2 | 2 | 100 |
| Shimada classification | ||||
| FH | 26 | 21 | 19 | 90.5 |
| UFH | 17 | 14 | 3 | 21.4 |
| Stage | ||||
| I | 12 | 9 | 8 | 88.9 |
| II | 6 | 5 | 5 | 100 |
| III | 3 | 3 | 3 | 100 |
| IV | 18 | 14 | 5 | 35.7 |
| IVs | 4 | 4 | 1 | 25 |
| Site | ||||
| Abdomen | 26 | 22 | 13 | 59.1 |
| Thorax | 13 | 9 | 6 | 66.7 |
| Other | 4 | 4 | 3 | 75 |
4. Discussion
PNTs are exceedingly rare tumors. The overall incidence of pediatric PNTs is not yet clear. In European children (0 - 14 years), the annual incidence was six cases per million (1). This was about 10.54 cases per million per year in the United States (16). However, PNTs are the most frequent solid tumors in children under five years of age. The occurrence of PNTs is unusual in adolescents and adults (17). In our series, 95% of the PNTs occurred in children under five years of age, and of those, half occurred in children less than one year old. Based on the literature (18, 19), girls are more likely to suffer from PNTs than boys are; however, in our research, there were slightly more boys than girls with PNTs (1.15: 1).
The clinical manifestations of PNTs are extremely variable are are correlated with primary tumor site, size, patient age, and the presence of metastasis. Some tumors have been found accidentally by ultrasound examination. PNTs that originate in the abdomen present as a mass with symptoms of abdominal pain or abdominal distension. Cervical and thoracic tumors may cause Horner syndrome and respiratory symptoms (20). Pelvic PNTs may cause sphincteral impairment (21). More rare symptoms include transverse myelopathy, hypertension, opsomyoclonus- ataxia syndrome, and treatment-resistant diarrhea (22-24). A total of 40% of patients may have metastatic dissemination of the tumor (25). Those patients may also have systemic symptoms (e.g., pallor, pain, weight loss, and/or fever) (26). In our study, the most common clinical symptoms of abdominal PNTs were abdominal pain or abdominal distension, followed by fever, which was present in five patients. The primary symptoms of thoracic PNTs was cough, which occurred in seven patients.
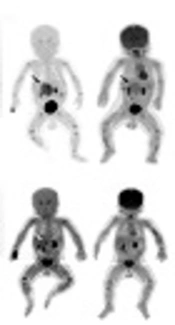
The US has employed a routine imaging tool and primary diagnostic method for the characterization of PNTs. CT or MRI may help to determine the tumor size, regional extension, lymph node involvement, and distant spread (16). Bone marrow aspiration and ECT (emission computed tomography) are performed to determine the bone marrow and bone involvement. Owing to high specificity and sensitivity in PNTs, especially in high-risk PNTs, meta-iodobenzylguanidine (I-MIBG) scintigraphy have been recommended as standard means of staging and response evaluation (27). The VMA level in the urine over 24 hours is valuable for the diagnosis of PNTs. VMA was elevated in most neuroblastoma cases, with a diagnostic sensitivity ranging from 66% to 100% (28). Increased levels of NSE, LDH, and ferritin may be helpful in the diagnosis and the prognostic classification of PNTs. In our series, I-MIBG scintigraphy was positive in 11 cases; meanwhile, urine VMA levels over 24 hours were elevated in 18 neuroblastoma cases and three ganglioneuroblastoma cases. Some patients with neuroblastomas had a high level of NSE and LDH, with no specificity. These indicators descended to normal within one month after operation.
Multiple strategies, such as surgery, chemotherapy, radiotherapy, autologous stem cell transplantation, and a combination of these therapies, have been used to treat PNTs (29-31). These strategies are combined on the basis of prognostic factors detected in the individual patient and the consequent risk-group assignment (32, 33). Surgery has an important role in treating PNTs, which may achieve complete tumor excision. In other cases, surgical biopsy is a safer choice for making a histological diagnosis in patients with PNTs that cannot be resected completely at one time. However, in experienced hands, minimally invasive surgery is a safe technique with a short hospitalization stay and minimal complications (34-36). In our series, 26 patients were open resected, while two patients underwent a laparoscopic surgery; the 15 remaining patients were biopsied. Chemotherapy is another important treatment for PNTs, especially in the case of neuroblastoma with metastatic and locally advanced disease. Preoperative chemotherapy can reduce the size of the tumor and can be helpful for radical resection. Postoperative chemotherapy is helpful for killing residual tumor cells. However, in our series, some patients gave up chemotherapy due to economic problems or due to the fact that the prognosis was bad.
The prognosis depended on the clinical stage of the disease, patient age at diagnosis, tumor biology (Shimada system), grade of tumor differentiation, MYCN oncogene amplification, 11q deletion, and DNA ploidy (37). Based on the criteria of the International Neuroblastoma Risk Group (INRG), neuroblastoma patients are currently subdivided into low-, intermediate-, and high-risk groups. The five-year survival rates are variable with low- (LR) to intermediate-risk (IR) cases, which typically have survival rates over 90%, and high-risk (HR) cases, with survival rates of only 40% - 50% (16). HR neuroblastoma is found in approximately 40% of cases and is associated with chemoresistance and tumor relapse (38). In our series, patients were difficult to follow up for a long period, which was due to various reasons. Therefore, we only obtained two year survival rates. The overall two year survival rate was 62.9%. Tumors with a high degree of malignancy, poor Shimada classification, high stage, or location in the abdomen may have had low survival rates, which was consistent with previous studies.
This study adds to the current knowledge of the diagnosis and management of PNTs. However, it is associated with some limitations. First, this study is a retrospective review, and it excludes certain prognostic indicators, for example, N-myc status. Second, the ganglioneuroblastoma and ganglioneuroma sample sizes are small. In addition, we have no long-term follow up data on the patients, and the overall recurrence, and survival rates are also unclear. Future studies with long-term follow-up are warranted to fully assess the safe and effective management of PNTs.
4.1. Conclusion
Pediatric PNTs originating from different sites have different clinical characteristics and outcomes. Imaging and laboratory data may be useful for the differentiation of PNTs. This study will serve as an aid for pediatric surgeons to be aware of the possible manifestations of PNTs in children.