1. Background
Although the incidence of preterm births has increased in the last few decades and affected approximately 11% of all pregnancies, neonatal mortality shows a decreased trend because of general use of mechanical ventilation and exogenous surfactant replacement therapy (1, 2). Subsequently, preterm birth has been the most common cause of death in early life (3). Some survivors of preterm birth were afflicted by prematurity-associated complications and sequelae in childhood and/or adulthood, such as developmental retardation, bronchopulmonary dysplasia (BPD) and congenital heart disease (CHD) (4-7). Moreover, population-based epidemiological data have shown close association between growth and development in early life and health and disease in childhood and/or as adults. Some evidence has suggested that administration and medical care in the post-neonatal period might benefit these surviving infants and ultimately promote their quality of life (8). Nevertheless, which life period during early life is the key period and determining its impact, remains unclear. Improved understanding of the early life period might help medical and public health personnel to make more reasonable decisions.
A new concept of life period from > 28 days to < 100 days after birth has been newly proposed as the post-newborn period, which renews the divides of infant age (9). According to the new concept, the early life aged less than 1 year should be divided into the neonatal period (≤ 28 days after birth), post-newborn period (from > 28 days to < 100 days), and modified infant period (from ≥ 100 days to 1 year). Meanwhile, the most important characteristics in the post-newborn period differing from the other two periods have been suggested: (1) different fatal diseases and mortality rate; (2) some diseases inherited from the newborn period requiring early and prompt treatment; (3) some peculiar diseases during this period requiring more attention; (4) either similar or different immune function; and (5) rapid growth and uneven development of organ systems. In the post-newborn period, infants should depend completely on breast milk except for some specific reasons. Evidence also supports the relationship between breastfeeding and health benefits in childhood and adults, including promoting long-term intestinal homeostasis (10), preventing gastrointestinal and respiratory tract infections (11, 12), decreasing risk of obesity and rheumatoid arthritis (13, 14), promoting receptive language intelligence and recognition development (15, 16), and regulating immune function (17). All these beneficial effects may be in virtue of some special matters in breast milk (18, 19). Establishment of the new concept may help to further reveal the nature of life, reduce infant mortality and improve the quality of life. It may also be useful to better understand the developmental origins of health and disease (8). Up until now, there has been no report referring to epidemiological data in the defined period.
2. Objectives
In the present study, mortality and etiology in a cohort of infants hospitalized over a ten-year period were surveyed and the differences were compared in the neonatal, post-newborn, and modified infant periods.
3. Methods
Public hospitals are divided into 3 grades: grade I, grade II, and grade III. A neonatal transfer collaboration network was initiated in 1999. In 2013, this network included 72 hospitals. Out of all of them, 12 hospitals belong to grade I, 30 to grade II, and 18 to grade III. The other 12 private hospitals which had a department of obstetrics and gynecology but did not have a department of pediatrics were excluded from the survey. All grade I hospitals were also excluded because they only provided basic medical care and all infants needing critical care had to be transferred to grade II and grade III hospitals.
Stratified randomized sampling was enforced in the selection of hospitals. One-third of grade II or grade III hospitals in each grade were included according to the stratified sampling method. A table of random numbers concealed in opaque envelopes was used to allocate and blindly randomize hospitals. After documenting the hospital’s consent, 10 grade II hospitals and 6 grade III hospitals were randomly selected to join the survey. Two hospitals were excluded from the survey for the following causes: one could not offer the necessary 10-year data, while another ultimately decided to withdraw from the survey. Finally, 14 hospitals were included in the surveillance.
The life period of an infant was divided into 3 continuous periods: neonatal period (0 - 28 days), post-newborn period (from > 28 days to < 100 days) and modified infant period (from ≥ 100 days to 1 year). The infants in the hospitals between January 1, 2004 and December 31, 2013 were included. Data of dead infants included birth weight (BW), gestational age (GA), gender, mode of delivery, mode of feeding, etiologies, and time of death were collected according to medical records.
The diagnoses of neonatal asphyxia, respiratory distress syndrome (RDS), pneumonia, encephalopathy of prematurity, retinopathy of prematurity, necrotizing enterocolitis, BPD and CHD were according to the manual of neonatal care (20). Sudden infant death syndrome (SIDS) was diagnosed according to the reported study (21). Preterm-associated disease (PAD) usually appeared as complications and sequelae of prematurity, which was used to assess the effects of prematurity for long-term prognosis (22). Here, it included four diseases: encephalopathy of prematurity, retinopathy of prematurity, necrotizing enterocolitis and BPD. Otherwise, the definition of breastfeeding means exclusively feeding with breast milk (no other food or drink) for the first six months after birth according to the World Health Organization (23).
3.1. Statistical Analysis
Categorical variables were compared using the χ2 test or the Fisher’s test. Differences in the primary and other categorical outcomes were estimated together with 95% confidence intervals. All analyses were carried out using computer software (SPSS 16.0 for Windows). A P value less than 0.05 was regarded as statistically significant.
4. Results
The survey was enforced in china between January 1,2004 and December 31, 2013. Overall, 155463 infants aged 0 - 1 year (61729, 21330, and 72404 in the neonatal, post-newborn and modified infant period respectively) were enrolled in the survey. The total gender (female: male) ratio was 85504: 69959. The gender (female: male) ratio was 34568: 27161 in neonatal, 11945: 9385 in post-newborn, and 38991: 33413 in modified infant period respectively. The total infant mortality was 6.16% (959/155463). Mortalities in the neonatal, post-newborn, and modified infant periods were 10.1% (623/61729), 6.9% (148/21330), and 2.6% (188/72404) respectively, which showed a significant difference (P = 0.000). In post hoc analysis, significant differences were found in 2010 (P = 0.012) and 2012 (P = 0.001) between neonatal and post-newborn periods, and from 2004 to 2013(P ≤ 0.001) between post-newborn and modified infant periods except for 2012 (P = 0.883).
Among these dead infants, 28.4% (272/959) were exclusively fed breast milk, 59.0% (566/959) ate formula and 12.6% (118/959) had mixed feeding. The total prevalence of exclusive breastfeeding was 27.3% (42401/155463), with 20.0% (12346/61729) in the neonatal period, 44.5% (9492/21330) in the post-newborn period and 28.4% (20563/72404) in the modified infant period, which also showed a significant difference (P = 0.000). The clinical characteristics of dead infants at birth included BW, GA, gender, mode of delivery, and mode of feeding which are shown in Table 1.
| Dead Infants | |
|---|---|
| BW, g | |
| < 2500 | 311 |
| ≥ 2500 and <4000 | 617 |
| ≥ 4000 | 31 |
| GA, weeks | |
| < 37 | 270 |
| ≥ 37 and < 42 | 677 |
| ≥ 42 | 12 |
| Gender | |
| Female | 419 |
| Male | 540 |
| Delivery | |
| Vaginal labor | 302 |
| Caesarean | 657 |
| Mode of feeding | |
| Pure breast | 272 |
| Formula | 566 |
| Mixed | 121 |
Clinical Characteristics of Dead Infants During 2004 - 2013
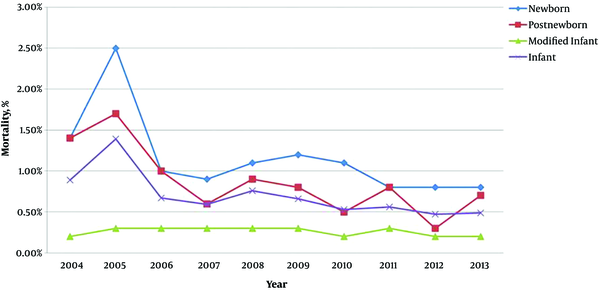
Figure 1 shows the changes in mortality during 2004 - 2013. The total infant mortality was decreased from 8.9% to 4.9%. Mortality in the neonatal period and post-newborn period was decreased by 42.8% [14.0% (42/2918) vs 8.0% (71/9385), P = 0.001] and 50.0% [14.0% (11/773) vs 7% (32/4448), P = 0.048] respectively, whereas mortality in the modified infant period remained at a similar level of about 2.0%. At the same time, yearly mortality showed a significant difference among the three periods (P ≤ 0.001).
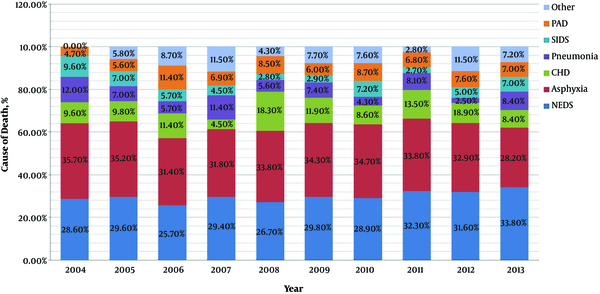
Figure 2 shows the changing trends of cause-specific mortality in the neonatal period from 2004 to 2013. Overall, RDS, neonatal asphyxia and CHD were three main causes of death. The proportion of death from asphyxia showed a gradually decreasing trend from 35.7% to 28.2%, but that from RDS indicated an inverse trend from 28.6% to 33.8%.
Trends in proportional contribution of the most common causes of death to the total numbers of death in neonatal period during 2004 - 2013. CHD: congenital heart disease. NRDS, neonatal respiratory distress syndrome; SIDS, sudden infant death syndrome; PAD, premature-associated disease including encephalopathy of prematurity, retinopathy of prematurity, necrotizing enterocolitis and bronchopulmonary dysplasia.
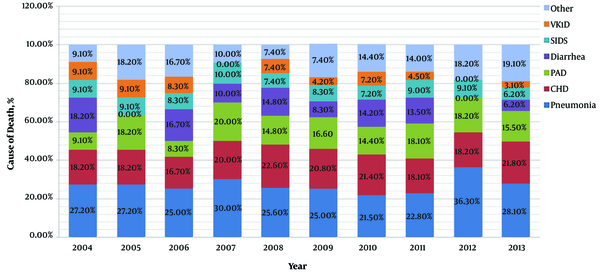
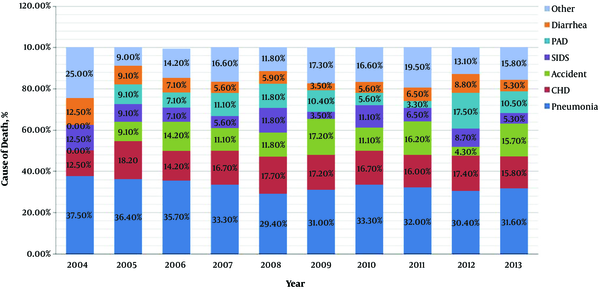
Figure 3 shows the changing trends of cause-specific mortality in the post-newborn period from 2004 to 2013. The three main causes of death in this period were pneumonia, CHD and PAD. During the 10 years, mortality of pneumonia and CHD were kept at a nearly stable level, but mortality of PAD showed an obvious increased trend from 9.1% to 15.5%.
Trends in proportional contribution of the most common causes of death to the total numbers of death in post-newborn period during 2004 - 2013. VK1D, vitamin K1 deficiency; SIDS, sudden infant death syndrome; PAD, premature-associated disease including encephalopathy of prematurity, retinopathy of prematurity, necrotizing enterocolitis and bronchopulmonary dysplasia; CHD, congenital heart disease.
Figure 4 shows the changing trends of cause-specific mortality in the modified infant period from 2004 to 2013. In this period, the three main causes of death were pneumonia, CHD and accidents. Although pneumonia and CHD still remained the two most common causes of death, accidents turned out to be the third most important cause of death instead of PAD in the post-newborn period.
Trends in proportional contribution of the most common causes of death to the total numbers of death in modified infant period during 2004 - 2013. PAD, premature-associated disease including encephalopathy of prematurity, retinopathy of prematurity, necrotizing enterocolitis and bronchopulmonary dysplasia; SIDS: sudden infant death syndrome; CHD, congenital heart disease.
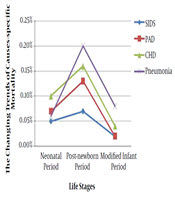
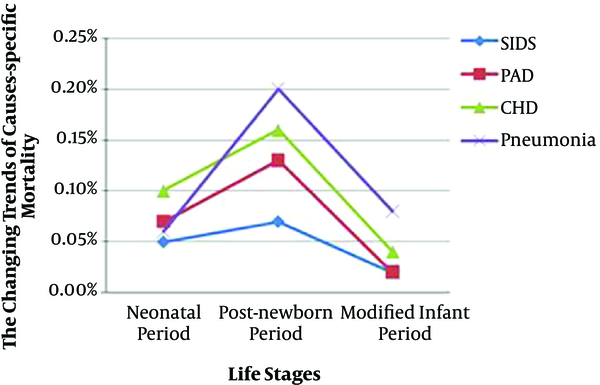
Some major causes of death appearing simultaneously in the neonatal, post-newborn, and modified infant period were compared and are shown in Table 2. There was a significant difference in mortality of PAD (0.7% vs 1.3% vs 0.2%, P = 0.000), pneumonia (0.6% vs 2.0% vs 0.8%, P = 0.000), CHD (1.0% vs 1.6% vs 0.4%, P = 0.000), and SIDS (0.5% vs 0.7% vs 0.2%, P = 0.001) among the three periods respectively. Longitudinally (from the neonatal period to the modified infant period), mortality of the four diseases was shown as an inverse “U” shape change, and the highest mortality appeared in the post-newborn period (Figure 5).
| Causes of Death | Neonatal Period | Post-Newborn Period | Infant Period | P Value | ||||
|---|---|---|---|---|---|---|---|---|
| Death (N = 623) | Hospitalization (N = 61729) | Death (N = 148) | Hospitalization (N = 21330) | Death (N = 188) | Hospitalization (N = 72404) | |||
| PAD, yes:No. (%) | 45:578 (7.8) | 45:61784 (0.7) | 27:121 (22.3) | 27:21303 (1.3) | 17:171 (10.0) | 17:72387 (0.2) | 0.000 | 0.000 |
| Pneumonia, yes:No. (%) | 38:585 (6.5) | 38:61691 (0.6) | 43:105 (41.0) | 43:21287 (2.0) | 61:127 (48.0) | 61:72343 (0.8) | 0.000 | 0.000 |
| CHD, yes:No. (%) | 63:560 (11.3) | 63:61666 (1.0) | 33:115 (33.0) | 33:21297 (1.6) | 31:157 (19.7) | 31:72373 (0.4) | 0.000 | 0.000 |
| SIDS, yes:No. (%) | 33:590 (5.6) | 33:61696 (0.5) | 14:134 (10.4) | 14:21316 (0.7) | 14:174 (8.0) | 14:72390 (0.2) | 0.139 | 0.001 |
Cause of Mortality During the Neonatal, Post-Newborn, and Modified Infant Period
Inverse “U” shape changes of the mortality of PAD, pneumonia, CHD, and SIDS. PAD, premature-associated disease including encephalopathy of prematurity, retinopathy of prematurity, necrotizing enterocolitis and bronchopulmonary dysplasia; SIDS, sudden infant death syndrome; CHD, congenital heart disease.
Effects of breastfeeding on the major causes of death appearing simultaneously in the neonatal, post-newborn, and modified infant period were compared and are shown in Table 3. Except for mortality of pneumonia (2.0 vs 2.2% vs 1.4%, P = 0.177), there was a significant difference in mortality of PAD (2.4% vs 1.2% vs 0.6%, P = 0.000), CHD (3.0% vs 1.6% vs 0.7%, P = 0.000), and SIDS (1.0% vs 0.9% vs 0.3%, P = 0.000) among the three periods.
| Neonatal Period | Post-Newborn Period | Infant Period | P Value | |||||
|---|---|---|---|---|---|---|---|---|
| Death (N = 623) | Breastfeeding (N = 12346) | Death (N = 148) | Breastfeeding (N = 9492) | Death (N = 188) | Breastfeeding (N = 20563) | |||
| PAD | 30 (4.8) | 30 (2.4) | 11 (7.4) | 11 (1.2) | 12 (6.4) | 12 (0.6) | 0.387 | 0.000 |
| Pneumonia | 25 (4.0) | 25 (2.0) | 21 (14.2) | 21 (2.2) | 28 (17.5) | 28 (1.4) | 0.000 | 0.177 |
| CHD | 37 (5.9) | 37 (3.0) | 15 (10.1) | 15 (1.6) | 15 (8.0) | 15 (0.7) | 0.166 | 0.000 |
| SIDS | 12 (1.9) | 12 (1.0) | 9 (6.1) | 9 (0.9) | 6 (3.2) | 6 (0.3) | 0.022 | 0.000 |
Effects of Breastfeeding on the Major Causes of Death During the Neonatal, Post-Newborn, and Modified Infant Perioda
5. Discussion
To obtain epidemiological data of infant mortality and etiology, the present multicenter ten-year surveillance was conducted. The results showed that mortality in the post-newborn period was found to be significantly different from that in neonatal period and modified infant period from 2004 to 2013. Meanwhile, the three main causes of death were different among the three periods. Moreover, four major causes of death appearing simultaneously in the three periods also showed significant differences. The major diseases including PAD, pneumonia, SIDS and CHD which showed inverse “U” shape changes from the neonatal period to the modified infant period, and the highest mortality appeared in the post-newborn period. To our knowledge, this is the first study on epidemiological data supporting the new concept of the post-newborn period.
Infant mortality has gradually dropped down with an obvious imbalance in different countries and areas (24-26). Neonatal mortality in the United States in 2006 has dropped by nearly half as compared with that in 1980 (from 8.48% to 4.45%) (27), whereas it has decreased by 70% in China in 2008 as compared with that in 1990 (from 34.0% to 10.2%) (28). A study referring to 11-year provincial-level time-series analyses in Mozambique showed that neonatal mortality in different provinces ranged from 13.6% to 4.2%, and the differences might be most strongly associated with institutional birth attendance, maternal and child nurse density and overall health workforce density (26). Infant mortality in developed areas including North America and Europe in 2002 dropped by half as compared with that in 1990 (from 14% to 7%), whereas it only dropped from 75% to 61% in developing areas. Meanwhile, the average mortality of the world was from 68% to 55% (24). In the United States, The infant mortality rate in 2013 was 5.96%, which did not change significantly from the rate in 2012 (29). In China, infant mortality has also obviously decreased in the past ten years. Compared with 24.1% in 2002, it accounted for 12.1% in 2012, and in 2013, it was down to 9.5%. In our cohort of 155463 infants, the neonatal and infant mortality decreased from 14% and 8.9% in 2004 to 8% and 4.9% in 2013, respectively. A survey enforced in 188 countries referring to 6.3 million children under 5 who died from 1970 to 2013 showed that compared with 33.4% in 1970 and 37.4% in 1990, neonatal deaths accounted for 41.6% of under-5 deaths, and would account for 44.9% in 2030 (30), and the trend indicated that neonatal deaths would play more and more important role during childhood.
The most interesting finding in the present study was that there was a significant different mortality rate among neonatal, post-newborn, and modified infant periods, and these differences mainly appeared between post-newborn and modified infant periods. In other words, there was more intimate association of causes of death between neonatal and post-newborn periods. Actually, the etiology of infant deaths varied from different countries to areas (31). In the worldwide, three main causes of 2.9 million annual neonatal deaths were attributed to preterm birth complications (34.5%), intrapartum conditions (24.1%) and infections (20.7%) (22). In developed countries, the three main causes of death in infants were congenital abnormalities, perinatal disease and SIDS (32-34), whereas in developing countries, they were neonatal disease, infection and congenital abnormalities (31, 35). In the United States, the most leading causes of infant deaths in 2013 were congenital abnormalities, low birth weight, maternal complications, and SIDS (29), while in China, they were birth asphyxia, preterm birth complications, congenital abnormalities and pneumonia (36). Data from 2007 - 2008 in Mozambique showed that 35% of deaths were attributable to bacterial sepsis, and 10% to complications of pregnancy, labor, and delivery during the neonatal period. Other major causes of death in neonates were fetal development disorders (6%), malaria (6%), hypoxia and asphyxia (6%), and pneumonia (4%) (37). The present survey showed that the three main causes of death in neonatal infants were RDS, neonatal asphyxia and CHD. The leading causes of death in the post-newborn period were different but had an intimate association with those in the neonatal period. During the 10-year study period, the mortality of neonatal asphyxia gradually decreased, which on one hand induced a higher survival rate in neonates, but on the other hand, might lead to more infants dying from PAD in the post-newborn period.
CHD was another important cause of death during the infant period, which accounted for nearly 28% of all major congenital anomalies (38). A meta-analysis in 2011 reported total CHD birth prevalence increased with significant geographical differences from 0.6 per 1,000 live births in 1930 to 9.1 per 1,000 live births after 1995. Among these infants with CHD, about 1% to 2% babies needed early treatment as soon as possible (39). In the present study, CHD-induced infant mortality was 0.8%, which was obviously higher than 0.12% in 2013 in the United States (29), and high death proportion of CHD in both neonatal (11.3%) and post-newborn (33.0%) periods indicated that these infants might not accept early detection and effective intervention (40). In this sense, pulse oximetry plus clinical assessment for detection of major CHD, especially critical CHD, might be feasible and reliable all over the world (41).
SIDS is defined as the sudden unexplained death of an infant younger than one year of age. SIDS usually occurs in a previously healthy infant with a thorough post mortem examination failing to demonstrate adequate causes of death (42). Overall, the rate of SIDS has decreased from 1.2 deaths per 1,000 live births in 1994 to 0.57 deaths per 1,000 live births in 2002 (43). In the United States, SIDS was the fourth leading cause of death in infancy, and it was about 0.43 per 1,000 infants in 2012, which showed a decrease with 0.40 per 1,000 infants in 2013 (29). Moreover, SIDS has been indicated to have a peak incidence at 2 to 4 months of age (44) In our survey, SIDS also showed inverse “U” shape changes in infancy, and the highest mortality appeared in the post-newborn period. Additionally, there were similar trends in PAD, pneumonia and CHD. The results strongly suggested that much attention should be paid to the newly proposed concept of post-newborn period in early life.
As is known, growth and development are the most important characteristics of infants and sensitive markers of their health, disease status and adequate nutrition (45), and breastfeeding is the most important nutritional factor in early life. Hence, to further verify the effects of a new concept on the mortality of the three periods, we also assessed the relations between the four diseases and breastfeeding. Our study demonstrated that, except for pneumonia, there were obvious associations between mortality of PAD, CHD and SIDS and breastfeeding among the three periods. We concluded that lower rate of breastfeeding might induce these differences. In 2013, an investigation referring to 90 Chinese cities showed that the prevalence of exclusive breastfeeding was 15.66%. Another report from the World Bank in 2008 indicated that the prevalence of exclusive breastfeeding was 67% in 1998, but decreased rapidly to 27.6% in 2008, with only 16% in big cities (46). These studies suggested a low rate and reduced trend of breastfeeding in China. Chung et al. reviewed the global breastfeeding rate as follows (47): In the USA, breastfeeding rate showed an increased trend from 2003 to 2009, and they were 36.0% and 16.3% at the ages of 3 and 6 months, respectively in 2009. In the same year in Korea, they were 50.0% and 11.4% at the two time points, respectively. In England, they were 17% and 12% in 2010. The highest rate appeared in Hungary with 95%. On average, almost 50% infants less than 3 months are breastfed. But, by 6 months, less than 25% are breastfed. In the present study, the prevalence of breastfeeding was 27.3%, with 20.0% in the neonatal period, 44.5% in the post-newborn period and 28.4% in the modified infant period. Our report of breastfeeding rate ranked in the middle and demonstrated an obvious reduction of breastfeeding rate in the modified infant period, which indicated the duration of breastfeeding was less than six months as recommended by the World Health Organization.
There is a total trend that more and more preterm newborns survive, and the gestational age of the survivors is also becoming smaller and smaller. Hence, neonatal asphyxia might lose the importance as the first cause of death. In addition to CHD or other congenital diseases, PAD would become the most common cause of death in the newborn and post-newborn periods in developing countries. A few survived infants suffered neurologic impairment and metabolic diseases in childhood and/or adulthood (48-50). These complications and sequelae would induce increased medical and social burdens and constitute a challenge for both public health organizations and healthcare providers. This survey might provide a new important insight into understanding the importance of early life in health and disease.
There are some limitations in the present study. Firstly, the selection of hospitals did not consider the difference of economy in different areas. Secondly, limited number of involved hospitals might mean some potential bias. These problems can be overcome by a national survey in the future. Given the potential limitations, future trials are needed.
In summary, the difference in mortality and etiology of the neonatal, post-newborn, and modified infant periods supports the concept of the post-newborn period. Further research is needed to better understand its clinical significance.