1. Background
Patent ductus arteriosus (PDA) is defined as the closure failure of ductus arteriosus after birth. Ductus arteriosus is normally essential in embryonic and fetal period because pulmonary vascular resistance is high and ductus arteriosus is necessary to inhibit the right ventricular failure. Few hours after birth, ductus arteriosus is closed functionally and gradually changed to ligamentum arteriosum.
PDA is more common in premature infants. It is more common in females with ratio of 3:1. It is diagnosed by physical examination, echocardiography and angiography.
Therapeutic approaches for an isolated PDA include medical (only in neonatal period), surgical, or percutaneous transcatheter closure.
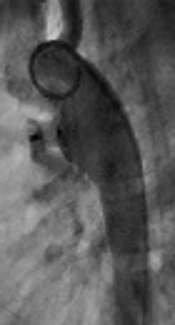
Cardiac angiography is the gold standard for assessment of PDA anatomy and size.
At present, percutaneous transcatheter closure is treatment of choice in patients with isolated PDA.
Successful device closure depends on accurate assessment of the size and morphology of the ductus.
The pulmonary artery end of the PDA has smooth muscles and can be stimulated and constricted in response to various pharmacological stimuli or catheter-induced spasm that may result in underestimation of the ductal size. Ductal spasm leads to improper device selection and inadvertent embolization of the device. In addition to the errors related to calibration, one also has to remember the possibility of ductal constriction when assessing the size of PDA by angiography. The aim of this study was to define if there is any correlation between the dimension of PDA measured by echocardiographic study and angiographic data. Such correlation may contribute to better selection of suitable device if any ductal spasm happens during interventional catheterization.
2. Methods
In this comparative study, we successively selected pediatric patients, who were referred for elective percutaneous PDA closure at Nemazee Hospital affiliated to Shiraz University of Medical Sciences (SUMS) since January 2016 till March 2017. Before the study, informed consent was obtained from the parents or guardians of all the children enrolled in this study.
Only patients with isolated PDA were enrolled in this study. All patients underwent full 2DE and 3DE study by PHILIPS machine (iE33) with probe (X5-1) before device closure on the day of angiography. PDA size measurements were done in short axis, high short axis and suprasternal views. Catheterization was done under conscious sedation and all patients received 100 U/Kg heparin after the venous and arterial lines were accessed. In catheterization laboratory after pulmonary and aortic pressure measurement, angiography was done on the lateral view and sometimes RAO or LAO views. We used 1.5 mL/Kg dye (Visipaque 300 mg Iodine/mL or Scanlux 300 mg Iodine/mL) with speed Wt × 1.5 mL/s.
After PDA size was measured and morphology defined, closure was tried by appropriate device.
Angiography and intervention were done by different physicians; however, all angiographic data measurements and 2DE and 3DE were done by the physician on charge. The data obtained by 3DE, 2DE and angiography were compared with each other. In 3DE, we used image rotation, Q lab or i-crop for evaluation of the size of pulmonic end, aortic end and length of PDA.
Data analysis was done by correlation formula in SPSS software, version 16.
3. Results
We had 26 patients with a mean age 28.73 ± 32.21 (range 6 - 121 months) and mean weight of 10.67 ± 5.2 Kg (range 5.2 - 25 Kg). The majority (84.6%) of our patients were female.
3.1. 2DE
The pulmonary end of PDA diameter was 3.65 ± 1.70 mm (range 1.18 - 7.52 mm).
The aortic end of PDA diameter was 6.43 ± 2.45 mm (range 2.53 - 12.2 mm).
The length of PDA diameter was 7.02 ± 2.17 mm (range 3.54 - 12.00 mm).
In our study, 8 patients (32%) had dilated LV (Z score ≥ 2 for BSA).
3.2. 3DE
The pulmonary end of PDA diameter was 3.57 ± 1.62 mm (range 1.36 - 7.50 mm). The aortic end of PDA diameter was 6.37 ± 2.15 mm (range 1.96 - 11.59 mm). The length of PDA diameter was 7.68 ± 2.28 mm (range 4.61 - 13.00 mm).
3.3. Angiography
The pulmonary end of PDA diameter was 3.63 ± 2.44 mm (range 0.9 - 9.20 mm). The aortic end of PDA diameter was 9.55 ± 3.05 mm (range 5.00 - 16.50 mm). The length of PDA diameter was 7.59 ± 2.97 mm (range 4.00 - 17.50 mm).
3.4. Pulmonic End of PDA
Comparison of data obtained from 2DE and 3DE revealed that the pulmonic end of PDA in 2DE, 3DE angiography well correlated with each other (2DE-3DE; r = 0.959, P-value < 0.001, 2DE-angiography; r = 0.875, P-value < 0.001 and 3DE-angiography; r = 0.841, P-value < 0.001).
3.5. Aortic End of PDA
There was no significant correlation between the aortic end of PDA in 2DE and 3DE with angiography (r = 0.219 and P-value = 0.293). There was no linear correlation between 2DE and 3DE with angiography. There was no significant difference between 2DE and 3DE. (2DE-3DE; r = 0.591, P-value = 0.002).
3.6. Length of PDA
Comparison of data obtained from 2DE and 3DE revealed that the length of PDA by 2DE, 3DE and angiography well correlated with each other (2DE-3DE; r = 0.771, P-value < 0.001, 2DE-angiography; r = 0.486, P-value = 0.014 and 3DE-angiograohy; r = 0.614, P-value = 0.001).
3.7. PDA Type
In this study, the majority of PDA shapes was compatible with type "A" by Krichenko et al. classification of anatomical types of patent ductus arteriosus (1).
3.8. Device Type
PDA closure was done by PDA occluder device, PFM coil or VSD occluder device. PDA occluder device type I was the most frequently used device.
In one patient, PDA was not closed due to the small size of the iliac vein and trauma to the vein; due to the need to a larger size delivery sheath, the patient was sent for surgical ligation and fortunately recovered without significant sequelae.
One of the patients developed ductal spasm during angiography. In this patient pulmonic end of PDA was 3 mm by echocardiography but aortogram in lateral view showed tiny PDA. Multiple tries from right side for passing through PDA was unsuccessful so, PDA was closed retrogradely by coil.
4. Discussion
Intermittent patency and closure of the ductus arteriosus was observed clinically, and this observation was uniquely demonstrated at the time of cardiac catheterization (2). Ductus arteriosus spasm may occur during aortogram or ductal closure and may result in inaccuracy of ductal measurement (3-6). This may lead to selection of undersized device for occlusion, with subsequent risk of device embolization (3, 4). While determination of the length and the ampulla of the PDA may be more accurate by 3D echocardiogram than 2D echocardiogram (42 patients) (7), Elsheikh et al. (25 patients) reported that the PDA anatomy, length and morphology, and both aortic and pulmonary ends can be better defined by real time three dimensional echocardiography (RT3DE) (8).
Color Doppler echocardiography significantly overestimates the minimum size of the patent ductus arteriosus; therefore, reconsideration of the respective size by other modalities is suggested (9). The size of PDA is defined clearly by 2D and 3D echocardiography. Because echocardiography data is measured in normal patient's status, the maximum size that is carefully measured is the actual size of PDA, especially when the cross area of PDA is measured in 3DE for both pulmonic and aortic ends.
Also, PDA measurement is reported by RT3DE; the main disadvantage of this modality was the time consumed during data analysis and image manipulation (8). However, in our study with the use of rotation and i-crop, the time consumed for determination of PDA size was decreased. Batlivala et al. mentioned that abrupt loss of a continuous murmur during catheterization confirms the diagnosis of ductal spasm, so they recommended routine examination of all patients prior to decision for selection of proper device for PDA closure (4). According to our study, the PDA size assessment by 2DE, 3DE and angiography well correlated and PDA spasm can be detected early by attention to 2DE and 3DE data. In one of our patients spasm of PDA was detected by comparison of echocardiography and angiography because size of pulmonic end of PDA was 3 mm by echocardiography and tiny during angiography.
The difference in aortic end dimension of PDA by 2DE and 3DE with angiography may be due to anatomical position of this site that is not clearly determined by echocardiography. It is mentionable that aortic size is important for some devices like pfm coil.
Statistical analysis of data of the patients below 24 months [20 (77%)] and above it revealed no difference in comparison with total data. Statistical analysis of data of the patients below 10 Kg [16 (61.5%)] and above it revealed no difference in comparison with total data.
Due to the significant correlation between 2DE and 3DE in pulmonic end and length of PDA with angiography, we may recommend routine and careful evaluation of all patients by echocardiographic study prior to the performance of catheterization for device closure. If there is any significant discrepancy between data of measurements, PDA spasm should be considered although it is a rare event.
4.1. Limitation
The limitations of this study include the number of patients in this series. Further studies are needed to define correlation of 2DE, 3DE and angiography. Also the small number of patients in this series does not allow for the identification of rare events.
4.2. Conclusion
The ductus dimensions measured by echocardiography and angiography well correlate with each other with respect to pulmonic end and length and are interchangeable. Such correlation may be helpful during percutaneous transcatheter occlusion if any ductal spasm occurs. 3DE has no significant advantage over 2DE in evaluation of PDA size for angiographic ductal closure. PDA closure based on echocardiography sizing is important especially for 3rd world centers with limited resource that cannot have most of PDA closure devices available on the shelf. Accordingly, we may suggest selection of a suitable device by maximum size of PDA that is measured by one of the 3DE, 2DE or angiography.