1. Background
Despite innovations and improvements in neonatal intensive care units (NICUs), respiratory failure still remains prognostically important in newborns (1). High-frequency oscillatory ventilation (HFOV) has been shown to result in less lung injury when compared to conventional mechanical ventilation (CV), especially in experimental studies (2). Cochrane meta-analysis shows that elective HFOV compared to CV results in a small reduction in the risk of bronchopulmonary dysplasia (BPD), but the evidence is weakened by the inconsistency across the trials. Probably many factors, related both to the intervention itself and to the individual patient, interact in complex ways (2).
In contrast to low benefits in elective use, HFOV as "rescue therapy" (rHFOV) with early and appropriate strategy in patients with progressive respiratory distress not responding to CV was shown to reduce the mortality and the frequency of extracorporeal membrane oxygenation (ECMO), shorten the length of hospital stay, and reduce the cost of the patients (1, 3, 4). However, there is only scant information on the application of rHFOV in the NICUs.
The aim of this study was to evaluate the effect rHFOV on mortality and morbidity in newborns who did not respond to CV, in order to try to list the factors affecting the outcome of the intervention and to identify the specific patient group, if possible, in which rHFOV is more successful.
2. Methods
All newborns who were admitted to the Ankara University School of Medicine NICU between 2012 and 2015 if not responding to CV, in case of progressive respiratory failure, and switched to rHFOV (n = 84) were enrolled in the study. All patients were treated with CV using volume-controlled ventilation before the institution of rHFOV. Tidal volume was kept below 7 mL/kg. Hypercarbia was tolerated if the arterial pH was above 7.25. HFOV was initiated because the FiO2 requirements of the patients exceeded 0.6 to maintain an arterial oxygen saturation of > 90%. A high-volume strategy consisting of incremental increases in the MAP until the arterial oxygen saturation of > 90%, a FiO2 of < 0.6, and with avoidance of lung overdistension was employed to patients.
The study was approved by the Ankara University School of Medicine Ethics Committee on 23.02.2015 with a report numbered 03-105-15, and patients’ data were collected from the patients’ files, which were kept in the hospital archive and hospital information management software (AviCenna ®) retrospectively.
Patients’ demographic data including gestational age (GA), birth weight (BW), gender, delivery mode, diagnoses on admission to the NICU, and pregnancy history were recorded.
Postnatal age at the time of intubation, surfactant history, duration of CV, postnatal age at the time of switch to rHFOV, blood gas analyses before starting and on follow-up (1st, 4th, and 24th hours of rHFOV), and duration of rHFOV were obtained. Patients were also checked for iNO and ECMO records. Total ventilation duration (CV+rHFOV), oxygen dependency at the time of discharge, morbidities such as BPD, retinopathy of prematurity (ROP), and grade III-IV intraventricular hemorrhage (IVH) were evaluated.
Patients were divided into two groups according to their response to rHFOV, as group S who survived and group D who died, and the groups were compared for their characteristics.
The analysis of the data was done in the SPSS 15 for Windows package program. The significance of the differences was investigated by t test and Mann Whitney test. Nominal variables were assessed by Pearson Chi-square or Fisher Exact test. GA and BW were tested according to the ROC curve analysis if they carried a distinctive feature for mortality. The threshold value according to the Youden Index was calculated for the variables with distinctive features. The value at the highest sensitivity and selectivity was determined as the threshold value. P < 0.05 was considered statistically significant for the analyses.
3. Results
3.1. Demographic Data
Among the newborns included in the study, male to female ratio was 2:1. The median GA of the patients was 32.5 (23 - 40) weeks and 50% (n = 42) of the patients were < 32-week preterm. Their median BW was 1675 (420 - 4370) g. Majority of the patients (82.1%) were delivered by cesarean sections (C/S). The median APGAR scores of the infants at the 1st and 5th minutes were five (0 - 9) and eight (2 - 10), respectively. 26.2% of the patients (n = 22) were the result of multiple gestations. The distribution of the patients according to initial diseases is shown in Table 1.
| Diagnosis | Patients, No. (%) |
|---|---|
| Respiratory distress syndrome (RDS) | 50 (59.5) |
| Sepsis | 8 (9.5) |
| Congenital pneumonia | 8 (9.5) |
| Persistent pulmonary hypertension (PPHT) | 7 (8.3) |
| Transient tachypnea of the newborn (TTN) | 4 (4.8) |
| Meconium aspiration syndrome (MAS) | 4 (4.8) |
| Pneumothorax | 1 (1.2) |
| Extra-pulmonary diseases | 2 (2.4) |
Diagnosis of the Newborns Who Received Rescue HFOV
The patients were intubated after a median postnatal age of 2.5 (0 - 600) hours. Although 64% (n = 32) of 50 patients with GA ≤ 34 weeks had received antenatal steroids, surfactant was given to 76.2% (n = 64) of the whole group. The patients were switched to the HFOV after a median of 28.5 (0 - 1248) hours on the CV.
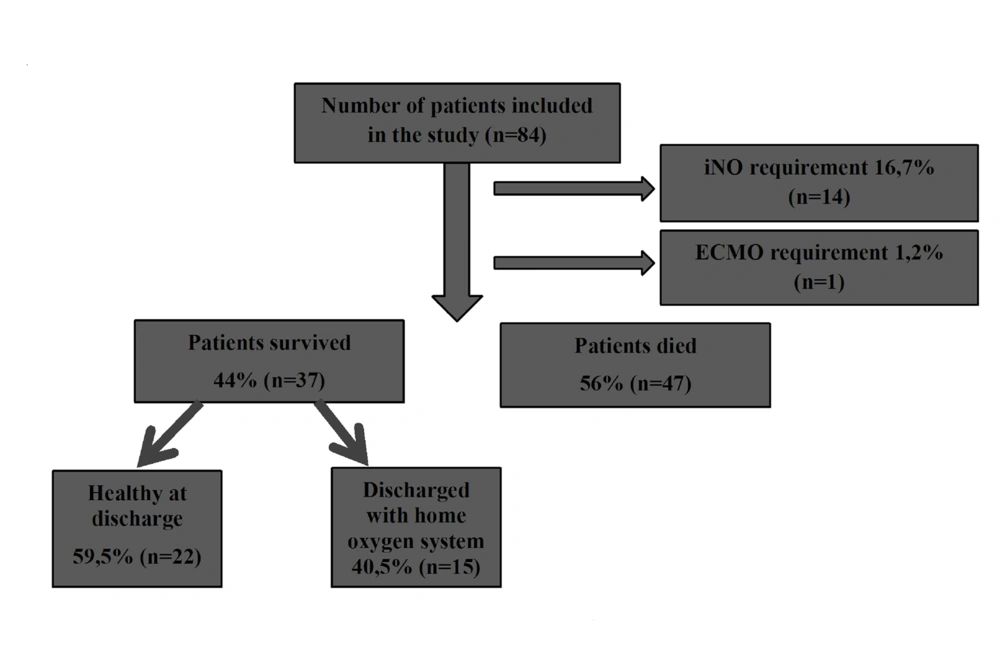
The initial rHFOV settings were mean MAP 13 ± 3 cm-H2O, frequency 10 ± 2, ΔP 30 ± 6, and median FiO2 100 (60 - 100) . The blood gases assessed at the follow-up are shown in Table 2. The median rHFOV duration was 35.5 (2 - 768) hours. 14 patients (16.7%) received iNO with a mean duration of 112.4 ± 60.4 hours. One patient (1.2%) underwent a successful veno-venous ECMO run for 192 hours (Figure 1) .
| pH | pCO2 | pO2 | SpO2 | |
|---|---|---|---|---|
| Initial | 7.13 | 59.2 | 53.9 | 85 |
| 1st hour | 7.20 | 47.1 | 57.8 | 93 |
| 4th hour | 7.25 | 41 | 63.3 | 96 |
| 24th hour | 7.31 | 41.9 | 61.9 | 97 |
The First 24-Hour Change in the Mean Blood Gas and Oxygen Saturation Values with HFOV Treatment
3.2. Complications and Mortality
Among the 84 patients, 56% (n = 47) died (group D) and 44% (n = 37) were discharged (group S) . Of the 37 patients who were discharged, 40.5% (n = 15) still needed oxygen at the time of discharge (Figure 1) .
BPD and ROP incidences were as 11.9% (n = 5) and 7.1% (n = 3) in 42 patients whose GA was below 32 weeks, respectively. The BPD rate increase was correlated with longer ventilation time and oxygen use (P = 0.001). Stage III-IV IVH was detected in 16.6% (n = 14) of the 84 patients under rHFOV. There was no significant difference in the duration of HFOV between patients with and without IVH (P = 0.37) .
3.3. Factors Affecting Mortality
3.3.1. Gestational Age
21% (n = 4) of the term babies with GA > 37 weeks (n = 19) died while 66.2% (n = 43) of the preterms with GA ≤ 37 weeks (n = 65) died. The risk of death in preterm infants was found to be 7.3 times higher (95% CI: 2.17 - 24.7, P = 0.0001). In terms of mortality, the threshold for GA was found to be ≤ 32.5 weeks with 75% specificity and 70% sensitivity (P < 0.0001).
3.3.2. Birth Weight
The mean BW of the infants in group D was 1345 ± 935 g in comparison with 2557 ± 1035 g in group S (P = 0.0001). 79.5% (n = 31) of the infants with BW ≤ 1500 g (n = 39) died while 35.6% (n = 16) of the infants with BW ≥ 1500 g (n = 45) died. The risk of mortality was 7 times higher in infants with BW ≤ 1500 g (P < 0.0001, 95% CI: 2.6 - 18.8). In terms of mortality, the threshold for BW was 1875 g with 75% specificity and 74% sensitivity.
3.3.3. HFOV Settings
The mean MAP setting at the beginning of rHFOV was found to be 13 ± 2.6 in group S and 13 ± 3.3 in group D. There was no statistically significant difference in the rHFOV initial settings between the groups (P = 0.06).
3.3.4. Blood Gas Analysis
Infants who had acidosis and hypercarbia in the pre-HFOV blood gases were found to have a higher mortality rate than patients without acidosis and hypercarbia. At the follow-up of the patients under rHFOV, blood gas examinations showed that acidosis and hypercarbia improved in one hour and oxygenation in four hours.
3.4. Subgroup Analysis for Patients Who Stayed on rHFOV for Over 24 Hours
Since the patients included in the study were switched to HFOV as rescue therapy and some of them died in the early period, statistical analyses were repeated in 48 patients (57.1%) who stayed on rHFOV for longer than 24 hours.
Mortality in preterm infants (48.1% vs. 15.4%) was found to be statistically higher (P = 0.03), and having a GA > 37 weeks increased the chance of survival up to 5.1 times (P = 0.03, 95% CI: 1 - 26.9). The threshold for GA was calculated as 32.5 weeks with 75.9% specificity and 57.9% sensitivity. BW was distinctive in terms of mortality also for patients staying on rHFOV over 24 hours (1378 ± 1120 vs. 2582 ± 1002 in groups D and S, respectively) (P < 0.0001). There was no statistically significant relationship between initial HFOV settings and mortality (P = 0.65).
4. Discussion
HFOV has been a hope for neonatal medicine seeking new ventilation strategies after high pressure, oxygen exposure, and open-collapsed alveolus-induced atelectotrauma in CV, which were found to be major factors leading to BPD in preterm infants. Any heterogeneity between the prior study results was not overcome and questions about the benefits and harms of HFOV in infants have remained unanswered.
Chen et al. (5) demonstrated that oxygenation indices of patients were better, and the ventilator settings in terms of MAP and FiO2 were lower in the HFOV group in their comparison of HFOV with CV applications with surfactant in infants with meconium aspiration syndrome (MAS). The duration of total ventilation, duration of oxygenation, and the length of hospital stay were also significantly shorter in the HFOV group. There was no significant difference between the two groups in terms of death and IVH; therefore, using early appropriate HFOV and surfactant application in MAS treatment was reported as an effective and safe method. Earlier studies in 1988 and 1990 reported that rHFOV might be useful in ECMO candidate newborns with irreversible respiratory failure (6, 7). Carter et al.'s study (7) showed that although there was no significant difference in terms of MV and hospitalization durations or mortality, bleeding disorders, convulsions, and renal failure were more frequently observed in ECMO patients than in the HFOV group. They stated that rHFOV might be preferred as the first-line approach in patients with progressive respiratory failure instead of direct ECMO application. The HIFO group (8) used HFOV on babies with RDS who had progressive respiratory distress under CV in 1993. This study found that in the rHFOV group, there was less pulmonary air leak than in the CV group but an increase was observed in the frequency of IVH. All of these studies on rHFOV have found to be controversial due to study designs, conditions, and limited patient numbers, in addition to that they took place long before the prevalence of current NICU practices such as antenatal steroids, surfactant, iNO, and open lung strategy in HFOV. Despite the opposing views on HFOV, the common consensus is that prospective randomized controlled rHFOV studies should be performed with the application of open lung strategy and current neonatal intensive care practices in a large patient population (9).
Recently, two randomized, controlled, multicenter studies in adult patient groups have led to the conclusion that rHFOV can increase mortality instead of reducing it and that other salvage methods should be developed as the cost of the patient greatly increased with HFOV (10, 11). Thereafter, two studies were conducted to see the effect of rHFOV in the pediatric age group. The first one was a retrospective study including 9177 patients, and in this study, mortality and duration of MV and NICU hospitalization were significantly lower in the CV group than in the HFOV group, similar to adult studies. Duration of MV and NICU hospitalization was found to be significantly shorter in patients who were switched to HFOV early than in patients who were switched later (12). The second study was a randomized controlled trial with a limited patient population to compare HFOV and CV in ARDS patients. As a result of this study, oxygenation in the HFOV group was better, but the results were disputed as the study included a very small group of patients (13). These studies in adult and pediatric patient groups have led to a re-questioning of the efficacy of rHFOV, but the heterogeneity of patient groups in these studies and the fact that HFOV has already been used on patients with critical clinical status have disputed the results of these studies (14).
In our study, we evaluated the results of the application of rHFOV to newborns with progressive respiratory failure under CV. The majority of our patient population was infants with RDS. As a result, almost half of the patients were discharged while 40.5% of them were in need of oxygen at discharge. Infants who had acidosis and hypercarbia in the pre-HFOV blood gases were found to have a higher mortality rate than patients without acidosis and hypercarbia. Dead infants had lower BW and GA than discharged infants, and prematurity and BW < 1500 g were associated with significantly increased mortality. There was no difference in initial HFOV settings between the groups. No statistically significant relationship was found between the duration of HFOV and IVH, ROP, and BPD.
In the light of these data, we found that the rescue HFOV was seven times more effective and increased the chance of survival when applied to newborns with GA > 32.5 weeks and BW > 1875 g. Due to the fact that no patient population in which HFOV is more successful has been identified in studies so far, we think that this may be a parameter when choosing patients to receive rHFOV. We believe that HFOV can be safely used as a salvage method in patients with progressive respiratory failure under CV since we did not establish any increase in the incidence of BPD, ROP, and IVH with rHFOV in our study. Although it is hard to conclude on the effect of HFOV on premature infant mortality, many of the patients receiving HFOV as rescue treatment responded to it, and no relationship between the incidences of prematurity-related morbidities in our study may give an idea for the safety of the treatment.
Recent data on the evidence that the use of elective HFOV compared with CV can result only in a small reduction in the risk of BPD (2), in addition to the ineffectiveness of HFOV in congenital diaphragmatic hernia patients in the prevention of ECMO (15, 16) have focused us on its rescue use in newborns. However, our study has limitations because it is a retrospective study with a small patient population. Although we did not have any control group and could not talk about the superiority or inferiority of HFOV over CV, we believe that a study with appropriate open lung method applied in HFOV, with standardized patient population using all current neonatal intensive care strategies, would give a correct result regarding rHFOV. In the light of our results, rHFOV may also be a less invasive step before ECMO at tertiary care centers and it can be used as an option to prevent the requirement of ECMO at centers without ECMO facilities. We believe that new improvements on data related to the HFOV use guided by transpulmonary pressure to prevent lung injury, its combination with iNO to have a better outcome on mortality, and its non-invasive use as nasal HFOV in severe cases should be followed (17-19).