1. Introduction
The gene encoding the potassium voltage-gated channel subfamily Q member 2, KCNQ2, is located on chromosome 20q13.3. This channel protein is 844-amino acid long, and its topological structure contains the “shaker-like” motif of six transmembrane domains (TMD), S1-S6, and a single P-loop (a pore-forming loop). The S4 TMD has been suggested to form the voltage sensor, and the P-loop contains the K+ pore signature sequence TxxTxGYG.1 Its homotetramer or heterogeneous KCNQ2/KCNQ3 complex mediates neuronal muscarinic currents (1, 2) playing an important role in the neuronal firing. Since Singh et al. (3) and Biervert et al. (4) first reported KCNQ2 mutations relevant to benign neonatal familiar convulsion in 1998 more than 80 mutations in KCNQ2 have been found in cases of epilepsy (2) and the clinical manifestations involving benign familial neonatal convulsion (BFNC) (3-6) epileptic encephalopathies, such as Ohtahara syndrome (7) and early myoclonic encephalopathy (EME) (8) are collectively referred to as epilepsies associated with KCNQ2 mutation. Different mutation types and locations are reported to be closely related to the clinical manifestation. The mutations are mainly located in the S1-S4 TMDs and the clinical manifestation is BNFC; the mutations located in the S5-S6 TMDs are closely related to encephalopathies.
Here, we report the case of an infant girl who was diagnosed with epilepsy associated with a KCNQ2 mutation, which could not be attributed to any reported epilepsy. This mutation in KCNQ2, identified using the next-generation sequencing, was de novo. More interestingly, the patient concurrently exhibited supraventricular tachycardia. Although there has not been any report regarding arrhythmia resulting from KCNQ2 mutations, considering that KCNQ2 plays an important role in electrophysiological processes, it is conceivable that the de novo KCNQ2 mutation may cause epilepsy and supraventricular tachycardia.
2. Case Presentation
The patient, a female infant, at three months of age, experienced generalized tonic-clonic seizures, with drooling and cyanosis, two times a day. The seizures lasted five min each time, and phenobarbital was administered. After the second relief, her heart rate raised to 200 beats/min, and real-time electrocardiography (EKG) hinted at supraventricular tachycardia. Luckily, the heart rate recovered to normal, without intervention, in a few minutes. Throughout the course, the patient did not have fever, vomiting, diarrhoea, or any other discomfort. She was admitted to our hospital after the seizures.
Regarding the patient’s medical history, she was hospitalized because of neonatal pneumonia, myocarditis, paroxysmal supraventricular tachycardia 13 days after birth. After the administration of anti-infection, anti-arrhythmic, and nutrition treatment, she recovered gradually, and she was discharged at 28-days-old. She was born through spontaneous full-term vaginal delivery without asphyxia, the Apgar score was unknown, the weight at birth was 3700 g, and breastfeeding was performed after birth. She raised her head steadily at two months of age, could laugh aloud, and see and hear well. Therefore, she was well developed before her first seizure. The history of anaphylaxis and family history were negative. General and nervous system examinations conducted during admission showed results matching with her age.
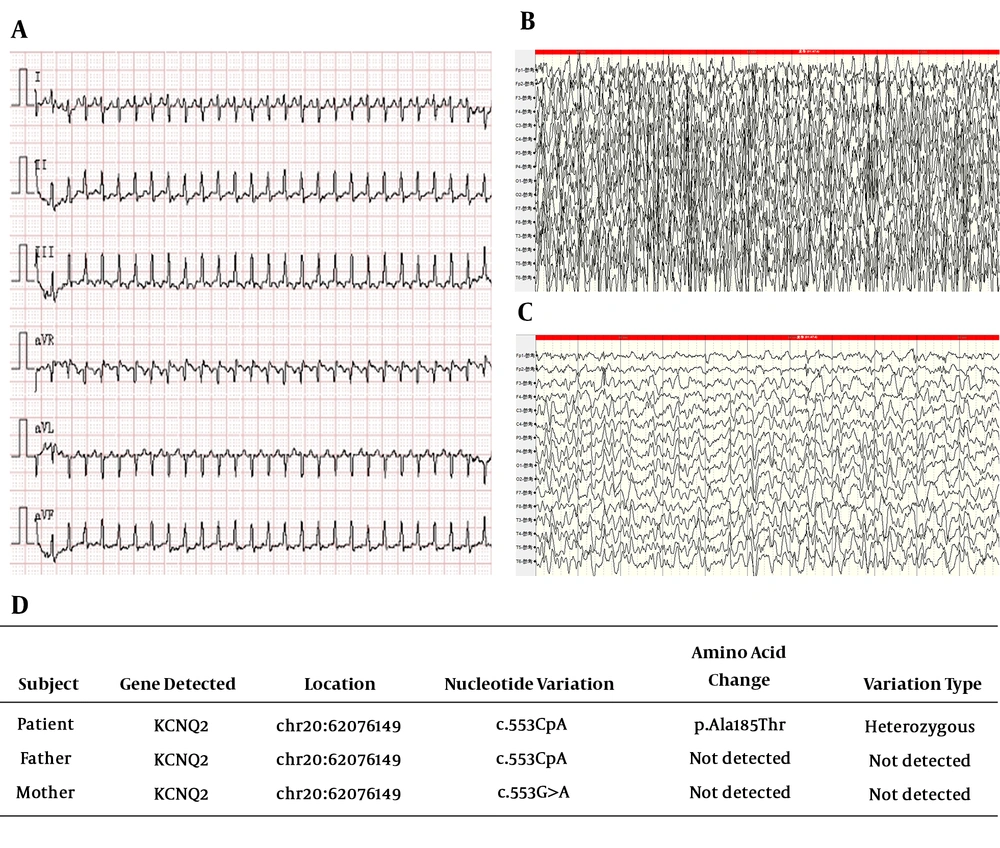
During this hospitalization, the patient experienced repeated generalized seizures without arrhythmia, and supraventricular tachycardia three times, confirmed using EKG (Figure 1A) and relieved through the stimulation of the vagus. She was diagnosed with epilepsy and supraventricular tachycardia, and levetiracetam and propafenone were administered for symptomatic treatment. Several days later, her condition was stable, and she was discharged from the hospital. On the fourth day after the first discharge, she was re-admitted to our paediatric intensive care units (PICU) because of frequent seizures with unconsciousness during the interictal periods. Due to unsatisfactory seizure control, sodium valproate was administered. After several days of observation, the convulsions were gradually controlled; the patient was discharged again and followed up monthly. Although the patient exhibited slow mental development in the first few days after frequent seizures, she recovered quickly, and her psychomotor development soon matched her age after the seizures were controlled. Further, the supraventricular tachycardia did not occur again, and therefore, propafenone administration stopped gradually. The patient has been followed up for one year, and she is 18-months-old now; the seizures have not recurred, and her development completely matches her age.
During hospitalization, a series of tests were performed. The results implied cytomegalovirus (CMV) infection (blood CMV-IgG and urine DNA were positive) and abnormal liver function. The results of cerebrospinal fluid examination (cerebrospinal bacterial smear and cultivation, fluid routine, biochemical test, screening for anti-viral antibodies, and autoimmune encephalitis antibodies) were normal. Cranial magnetic resonance imaging (MRI) result was also normal, and the results for blood tandem mass spectrometry and urine gas chromatography-mass spectrometry were negative. During video EEG recording, the patient experienced generalized tonic-clonic seizures three times. Ictal EEG showed a generalized spike, spike-slow, and slow waves (Figure 1B and 1C). Metabolic and structural bases of aetiology were not considered because of the above test results. Infective reasons could not be precluded because of the aforementioned CMV infection. Therefore, we further made PCR technique to validate, and the results showed negative CMV DNA in the cerebrospinal fluid. Considering the age at seizure onset and the frequency of seizures, we conceived that genetic mutation might be the cause of epilepsy. Therefore, we performed a genetic test after obtaining her family’s consent. Her genetic analysis report implied a de novo mutation in KCNQ2 (c.G553>A [p.Ala185>Thr]) (Figure 1D), which was not found in her parents, and had not been reported before. The mutation of KCNQ2 is autosomal dominant; hence, heterozygous mutation of KCNQ2 might lead to the disease.
3. Discussion
Epilepsy associated with KCNQ2 mutation, as the name implies, is epilepsy resulting from the mutation of KCNQ2. Due to the mutations such as missense, non-sense, truncations, splice-site defects, and frame-shift mutations, as well as sub-microscopic deletions or duplications, clinical manifestations such as BFNC, EME, and early-onset epileptic encephalopathy may occur.
Benign familial/non-familial infantile epilepsy, similar to BFNC, is an epilepsy syndrome with infantile onset. It is inherited in an autosomal dominant manner, with typical characters including the infantile onset of focal seizures, and the seizures may be frequent with intractable onset but can be spontaneously resolved. The patients exhibit normal psychomotor development, normal interictal EEG, and cranial MRI, with or without a family history. Moreover, about > 90% of such cases involve the mutation of PRRT2. Further, SCN2A, KCNQ2, and KCNQ3 mutations are also reported in BFNC.
In the present case, the patient experienced her first seizures in the infantile period (3-months-old), with the frequent onset of seizures (about 2 - 3 times a day), and she showed normal results for interictal EEG and cranial MRI. However, she exhibited generalized tonic-clonic seizures and status epilepticus, followed by slow psychomotor development. Moreover, the seizures were controlled gradually after valproate was administered (unlike intractable seizures).
Gene reports showed a de novo heterozygous mutation of the KCNQ2 gene (NM_172107.2): c.G553>A [p.Ala185>Thr] by whole-exome sequencing, which was confirmed by Sanger sequencing. The results of the software for the prediction of pathogenicity for this novel mutation p.Ala185>Thr is: deleterious, SIFT (http://sift.jcvi.org/); probably damaging, Polyphen2 (http://genetics.bwh.harvard.edu/pph2/); and disease-causing, Mutation Taster (http://www.mutationtaster.org/).
By combining the clinical manifestations and the gene report, we diagnosed the patient with epilepsy associated with KCNQ2, but the subtype could not be attributed to any reported epilepsy. Although the patient exhibited infantile onset of seizures, the seizure type does not match the BFNC (the typical character of BFNC is the onset of focal seizures in the infantile period, without status epilepticus or affected psychomotor development). After frequent seizures, the patient exhibited slow mental development in the first few days, but recovered quickly, and her psychomotor development soon matched her age. Therefore, the observed symptoms are part of a benign process and are not completely in accordance with epileptic encephalopathy. Moreover, the seizure was sensitive to the administration of valproic acid, and the patient showed normal cranial MRI and interictal EEG results. All the above features imply that this may be a new subtype of epilepsy associated with KCNQ2.
Another observation in this case was the concurrence of supraventricular tachycardia (SVT). Cardiac channelopathies can be misdiagnosed as refractory epilepsy while in fact, these events represent convulsive syncopes (9, 10). Heron et al. (10) reported the case of a patient with electrophysiologically verified neonatal long QT syndrome (LQTS) and neonatal seizures in the presence of a controlled cardiac rhythm. They tested some genes associated with LQTS (SCN5A and KCNE2) and benign neonatal familial convulsion (BNFS) (KCNQ2 and KCNQ3), and performed comparative genome hybridization; a de novo mutation was found in SCN5A [c.4868G>A (p.R1623Q)], and it was likely to be the primary pathogenic event. Therefore, the authors considered a pathophysiologic mechanism by which the combination of molecular changes might have caused seizures. In the present case, although the seizure and arrhythmia coincided, ictal EEG did not coincide with EKG arrhythmia; hence, we preclude the mechanism of seizures caused by supraventricular tachycardia. Moreover, the arrhythmia did not recur after the seizures were controlled. Considering that KCNQ (Kv7) is distributed in the brain and heart, we speculate that probably this mutation in KCNQ2 affected the above two organs, which has been called cardiocerebral channelopathy. However, this is the first report of a patient with concurrent epilepsy associated with KCNQ2 and SVT, and we should found more cases coincident in the above two events. More work should be done to test the KCNQ2 protein expression and functional study about the de novo mutation in our following days.
The de novo mutation in KCNQ2 [c.553G > A; p.(Ala185Thr)] resulted in benign infantile epilepsy, and the concurrence with arrhythmia may be linked with this mutation.