1. Introduction
Dieulafoy lesion (DL) is mucosal erosion into a caliber-persistent submucosal artery (1). The lesion is named after G. Dieulafoy, who initially reported it in 1898. DL is a rare cause of gastrointestinal (GI) hemorrhage, reported to be around 1-2% of acute or chronic episodes of GI bleeding (2). It usually causes massive GI bleeding and is difficult to diagnose (2, 3).
DL is typically located in the gastric fundus, and it usually occurs in adults, especially in the males and the elderly (3-5). It is an extremely rare source of GI bleeding in children. According to the reports, DL may occur as a possible congenital cause in children (6).
Here, we report an actively bleeding jejunal DL in a male adolescent. The case was finally diagnosed by intraoperative endoscopy and successfully treated by surgical resection. To our knowledge, this was the first report of small bowel DL diagnosed by intraoperative endoscopy in a child.
2. Case Presentation
A 15-year-old boy was admitted to the hospital with hematochezia and hypotension. He suffered from epigastric pain, vomiting, and a large amount of hematochezia. Neither peptic ulcer, nor bleeding disorders were found in the patient or in his family’s medical history. Upon arrival in the emergency department, the patient was anemic and ill-looking with complaints of dizziness and dyspnea. His temperature was 36.7°C, his pulse rate 135/minutes, his respiratory rate 40/minutes, and his blood pressure was 88/38 mm-Hg. On the physical examination, the patient had pale conjunctiva, bounding pulse, and a slightly distended abdomen without tenderness. The liver and spleen were not enlarged. The patient was diagnosed with impending hemorrhagic shock due to massive lower GI bleeding, and was transferred to pediatric intensive care unit.
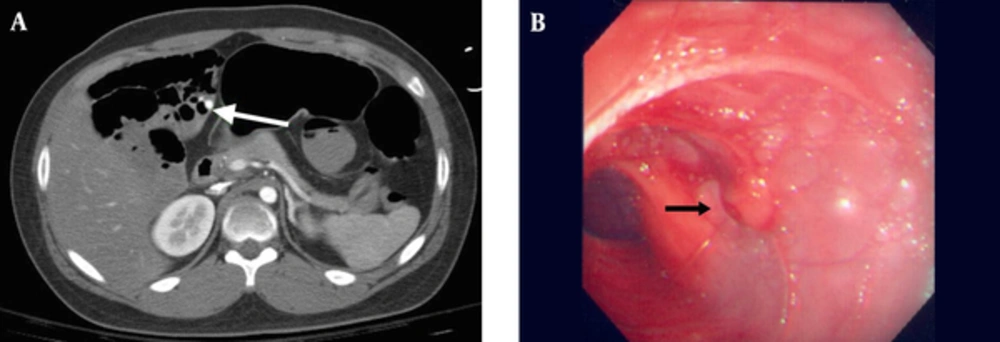
Hemoglobin, hematocrit, and platelet were 8.1 gm/dL, 25.2% and 116,000/mm, respectively. Serum transaminases, bilirubin, blood urea nitrogen, creatinine, prothrombin time, partial thromboplastin time, bleeding time, and electrolytes were all normal. The rectal bleeding continued over the next hours. The follow-up values of hemoglobin, hematocrit, and platelet remained low (8.3 gm/dL, 25.7%, and 104,000/mm, respectively) after transfusion of two units of packed red blood cells, nine units of platelets, and two units of fresh frozen plasma (FFP). Endoscopy and colonoscopy were performed. The endoscopy showed only anemic gastric and duodenal mucosa. The colonoscopy revealed fresh melenic stools covering the mucosa of colon, cecum and terminal ileum; otherwise, an edematous terminal ileum with scattered polypoid nodular lesions (lymphoid hyperplasia) was observed. The patient received additional four units of packed red blood cells, seven units of platelets, and two units of FFP during the endoscopic examinations. Double-balloon enteroscopy was performed via anus to detect any suspicious lower GI bleeding. However, no active bleeders were found at rectum, colon, and ileum. A subsequent computed tomography (CT) angiography revealed diffuse dilatation of small bowel, colon and rectum, with much blood retention. We observed two contagious well-enhanced nodular lesions at the right upper quadrant of the abdomen on the arterial phase, and we also noticed a leakage of contrast medium into the jejunum on the delayed phase. Therefore, we suspected that the lesions had active bleeding arising from the mucosal surface of jejunum (Figure 1A).
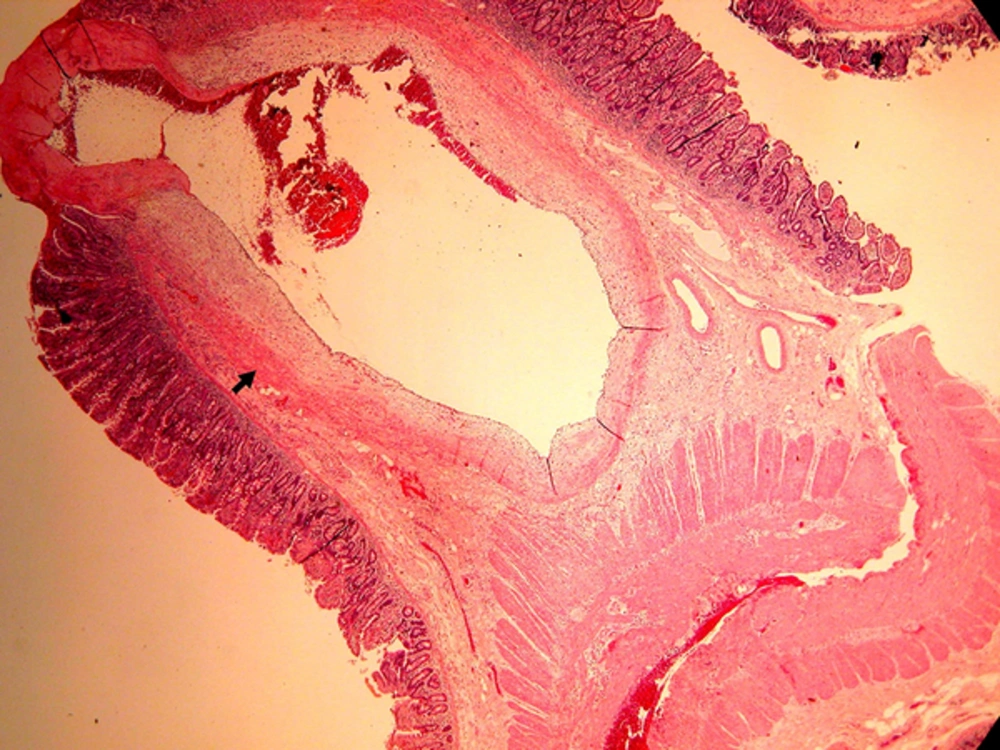
The patient’s hemoglobin level dropped to 4.5 mg/dL. He underwent emergency surgical laparotomy. At operation, a small incision of terminal ileum was performed to explore the bleeders by flexible endoscopy. After aggressive endoscopic irrigation of fresh blood and blood clots with normal saline fluid, we found no active bleeder in the ileum. We made another small incision near jejunal-ileal junction, and found a mucosal protruding vascular lesion (approximately 1 cm in diameter), with pulsatile spurting bleeding at distal jejunum approximately 125 cm from the Triet’z ligament. After closing the two incisions of ileum, partial bowel resection was performed, and the resected piece revealed that the lesion was a caliber-persistent submucosal dilated artery, approximately 5 mm in diameter, which was protruding 3-4 mm from a non-ulcerated mucosal surface (Figure 1B). The patient received four units of packed red blood cells, four units of platelets, and two units of FPP at operation. Histopathology revealed a Dieulafoy lesion of the jejunum. A tortuous, dilated and thick-walled vessel in the submucosa of the small bowel, measuring 5 mm in diameter was protruded through a small mucosal defect without surrounding inflammatory reaction (Figure 2).
The patient’s vital signs became stabilized after operation; his hemoglobin and platelet values rose to 11.5 mg/dL and 167,000/mm after transfusion of six units of packed red blood cells and four units of FFP. The patient was advanced to a regular diet two days later and discharged five days after the operation. There was no recurrence of bleeding in the follow-up period.
3. Discussion
DL is most often found in the stomach (71 %), followed by duodenum (15 %) and esophagus (8 %). It is uncommon in jejunum, ileum, colon, and rectum (4). DL was observed to be associated with the usage of NSAIDs or anticoagulants, or conditions of alcoholism, stress, or cardiac/pulmonary failure in the elderly (4, 5). Mucosal erosion or ischemic injury, which is possibly related to aging and cardiovascular diseases, further weakens the overlying mucosa.
The consensus suggested that DL might rip spontaneously, causing a major bleeding due to the overlying pulsating artery eroded progressively by the mechanical pressure from the abnormal vessel. Histologically, DL is a large three-layered artery with medial hypertrophy, but normal architecture without inflammation, deep ulcerations, and penetration of the muscularis propria, vasculitis, aneurysm formation, or atherosclerosis (1). There was no atherosclerosis or its associated risk factors in our case.
Characteristically, bleeding DLs are presented as acute or recurrent massive GI hemorrhage; there are usually no symptoms of anorexia, dyspepsia, or abdominal pain. The presentation of massive rectal bleeding in our patient was consistent with the reported cases of bleeding DLs in the small bowel (7). Preceding illness of epigastric pain and nausea in our patient might have been due to the large amount of blood retained in the intestines, and an ischemic effect of GI tract by massive blood loss.
Patients with bleeding DL usually present with anemia and postural hypotension, and their mean hemoglobin level on admission has been reported to be between 8.4 - 9.2 g/dL (8). The average transfusion requirement for the initial resuscitation is usually 3 to 8 units of packed red blood cells (8). Our patient had persistent anemia and hypotension at admission, and needed a major blood transfusion (16 packed red blood cells, 13 units of platelets, and 8 units of FFP within 24 hours) to reach hemodynamic stability. The need for the major blood transfusion in our case was due to a giant DL (0.5 cm-diameter of submucosal artery), with acute massive bleeding.
Our case experience confirmed that the diagnosis of patients with DL of the small bowel is often delayed due to inaccessibility and difficulty in localizing the bleeding site. Multidisciplinary approaches such as abdominal CT, radionuclide scan, angiography, capsule endoscopy, and balloon-assisted enteroscopy may help localize the source of bleeding of the small bowel. Angiography is helpful for those patients whose initial endoscopy have failed to detect the bleeding source, while the patient’s bleeding is active. The diagnosis of DL of the small intestine can be made angiographically (2, 9). The three-phase helical CT angiography could help identify the location of acute lower GI bleeding (10). The diagnosis of the bleeding site was established by CT angiography in 79.2 % (19/24) of the patients (9). The detection of active bleeder by CT angiography was made by observing the contrast enhancement of the bowel wall to evaluate the vascular extravasations of the contrast medium, or thickening of the bowel wall, polyp or tumor, and vascular dilation. In our case, CT angiography clearly identified the active bleeders by observing the enhancement of contrast media during arterial and delayed phases. Small bowel bleeding could be diagnosed and treated directly with the development of balloon-assisted enteroscopy (11, 12). In our case, balloon-assisted enteroscopy failed to detect the bleeders due to continual active hemorrhage with massive blood clots retained in the small bowel.
If the endoscopic and angiographic approaches fail, surgery must be considered taking into account the patient's clinical condition within an appropriate time. Intraoperative enteroscopy remains a valuable tool in patients with obscure GI bleeding, and it directly evaluates the small intestine, attains a high diagnostic outcome and performs an endoscopic and/or surgical treatment in patients with small bowel hemorrhage (13, 14). Consistent with previous experiences (15), DL of the small bowel in our patient could be clearly observed by intraoperative enteroscopy, using flexible endoscope by the incisions of small bowel.
To our knowledge, there were approximately 30 reported cases with jejunal DL based on the literature review, of which only four were about pediatric patients (14, 16, 17). Adult case series reported successful hemostasis with balloon-assisted enteroscopy in patients with DL of the small bowel (11, 12). Dulic-Lakovic et al. reported rebleeding in 30% (3/10) of the patients undergoing balloon-assisted enteroscopy, with 20% (2/10) eventually needing surgical intervention (11). A recent case series (n = 8) indicated that balloon-assisted enteroscopy achieved 100% hemostasis of small bowel DL with low (12.5%) rate of rebleeding and avoidance of surgery (18). Bleeding DL of the small bowel has been successfully treated by embolization in pediatric patients (19, 20). This treatment is usually reserved for patients who do not wish to undergo endoscopic therapy and are poor candidates for surgery.
In a majority of the adult cases, endoscopic hemostasis with balloon-assisted enteroscopy can replace surgery (11-13), while pediatric patients are usually treated with embolization or surgery (16, 17, 19). In our case, surgery with intraoperative endoscopy was the last and best treatment option due to failure of identifying the bleeder with balloon-assisted enteroscopy and deteriorated hemodynamics. A huge actively bleeding DL indentified by intraoperative endoscopy at jejunum is critical for directing surgical therapy.
3.1. Conclusions
CT angiography and further surgical intervention with the assistance of intraoperative endoscopy help diagnose the bleeding DL in the small intestine after negative exploration of bleeders in endoscopy, colonoscopy, and double-balloon enteroscopy. Additional experience with intraoperative endoscopy is a relatively safe and efficient modality for evaluating the bleeding DL of the small intestine.
3.2. Consent
Written informed consent was obtained from the patients for publication of this case report and accompanying images.
3.3. Ethics
Institutional Review Board of the human research committee at Chang Gung medical foundation approved the study protocol (No.: 201600485B0).