Dear Editor,
Clinical manifestations of adrenal hemorrhage (AH) vary from asymptomatic newborns to life-threatening hypovolemic shock and adrenal crisis (1, 2). In this report we represent a cyanotic term infant, initially suspected of cyanotic congenital heart disease (CCHD) due to persistent oxygen desaturations and hypotension, later diagnosed of massive unilateral hemorrhage resulting in hypovolemic shock and relative adrenal insufficiency.
A 2940-gram term baby was given birth by prolonged spontaneous vaginal delivery at another center (one and five minute Apgar scores of three and six). She was intubated, received surfactant and transferred to our facility for further diagnosis of suspected CCHD due to persistent cyanosis.
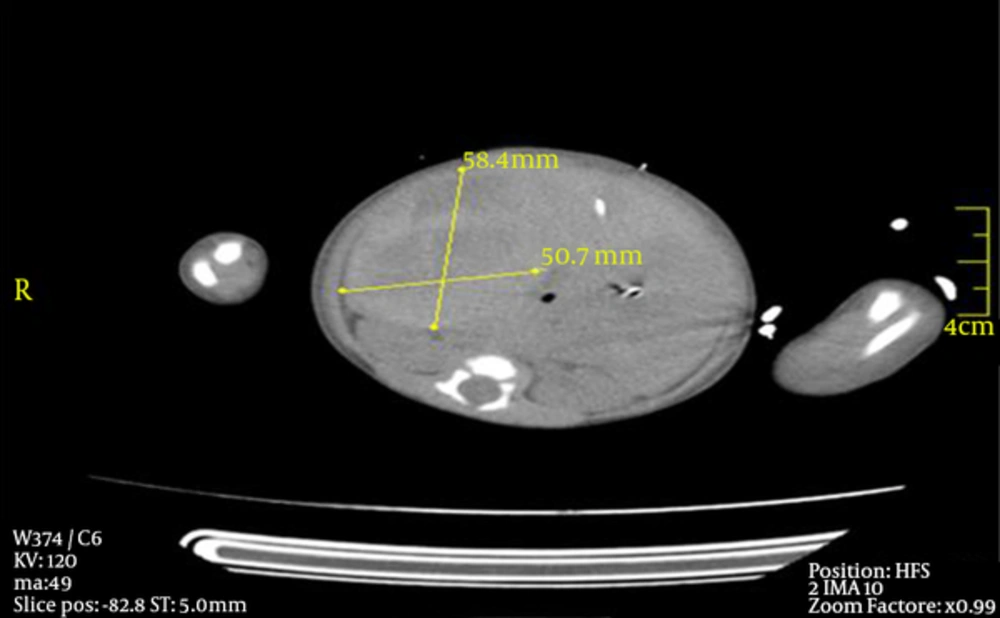
At arrival (14 hours after delivery), physical examination revealed severe ecchymotic lesions on her body, palpable abdominal mass on the right quadrant, pulse oxygen saturation 78% (on 100% FiO2), hypotension (mean blood pressure 29 mmHg), murmurs at mesocardiac area with positive femoral pulses. Clinical deterioration occurred rapidly; severe decline in hemoglobin from 15.5g/dL (initial level at previous center) to 4.4 g/dL and metabolic acidosis (arterial blood gas pH: 6.9, HCO3:10, BE: -18) was detected. Biochemical results did not reveal abnormality (including electrolytes and glucose) except alanine aminotransferase (ALT) 588 U/L and creatinine-kinase 530 mg/dL. Despite fluid resuscitation (fluids, erythrocyte suspensions and free frozen plasma) and inotropic support (both dopamine and dobutamine 10 µg/kg/min), inotrope-resistant hypotension persisted leading to initiation of hydrocortisone (50 mg/m2). Basal ACTH and cortisol levels obtained before hydrocortisone were normal (ACTH: 35 pg/ml, N < 46 pg/mL; serum cortisol: 12.06 mcg/dL, N:7-29 mcg/dL). Mild patent ductus arteriosus was detected on echocardiography (no sign of CCHD). Ultrasound revealed 65 × 57 mm sized capsulated hematoma on the right adrenal gland, later confirmed by abdominal tomography (hematoma with 58.4 × 50.7 mm size was compressing abdominal aorta and gall bladder) (Figure 1). She necessitated high frequency oscillatory ventilation due to thrombocytopenia (platelets: 41 × 103/μL) related bleeding into the lungs. Probable causes of coagulation diathesis and thrombocytopenia were ruled out by pediatric hematologists; asphyxia and prolonged labor seemed to be the cause of hematoma. Hydrocortisone was discontinued on day six following restoration of cardiovascular hemodynamics. After nine days of ventilation, she was discharged from our unit on day 19. Serial ultrasounds demonstrated complete resolution at 11 weeks without any calcifications (day 9: 70 × 40 mm; day 18: 48 × 39 mm; 4th week: 32 × 20 mm, 11th week: complete resolution).
High vascular nature and relatively large size makes the adrenal gland vulnerable to hemorrhage (1). It is usually unilateral (right adrenal gland) within the subcapsular area and needs at least 90% organ destruction to cause adrenal insufficiency in delayed phases (3, 4). Etiology consists of hypoxia, traumatic delivery, septicemia and bleeding diathesis (3). We believe the underlying cause of hematoma was prolonged labor and asphyxia; it was unilateral but massively compressing the nearby tissues and caused hemodynamic imbalance which mimicked CCHD. Interestingly, despite the inotrope-resistant hypotension requiring hydrocortisone replacement, the hypothalamic-adrenal axis and adrenal functions were within normal ranges. As well known, cortisol concentrations should be much higher during stress, but relative adrenal insufficiency can be observed in term newborns and is clearly associated with cardiovascular dysfunction (5). Fernandez et al. reported high prevalence of low cortisol values in critically ill, hypotensive, term newborn infants (6). The sudden withdrawal of CRH from placenta, transiently suppressed hypothalamic CRH production or the refractory stimuli for pituitary gland to produce ACTH contributes to relative adrenal insufficiency (5). Our patient’s persistent hypotension in absence of electrolyte/glucose imbalance might be due to relative insufficiency and hydrocortisone administration for six days probably masked the clinical and laboratory characteristics of significant insufficiency. Since adrenal crisis is transient and responds well to steroid replacement therapy (7), the patient did not show any signs of insufficiency in either hospitalization period or outpatient visits.
Adrenal Hemorrhage mostly resolves between three weeks to six months (3). Ultrasound is the choice of follow-up to monitor the multiloculated cystic masses, calcifications and the resolution (3, 8). Similarly we observed complete resolution in eleven weeks without any calcifications.
In a cyanotic infant with prolonged labor, CCHD might not always count in diagnosis; severity of the clinical representation of hypovolemic shock can be so devastating that it might be misdiagnosed as heart disease and the normal values of electrolytes or hormones do not rule out the relative adrenal insufficiency.