1. Background
Nephrotic syndrome is associated with an atherogenic lipoprotein profile of increased total plasma cholesterol and low-density lipoprotein (LDL) (1, 2). Children with steroid-resistant nephrotic syndrome (SRNS) are chronically exposed to abnormal lipid levels. Also, high serum lipid level in adults is an established risk factor for atherosclerosis (3), cardiovascular diseases (CVDs), and even cardiac death (4).
Adult patients with SRNS have been shown to experience more than a 5-fold increase in the relative risk of myocardial infarction (MI), as well as a significant rise in the relative risk of coronary death within 5.6 years of follow-up (4). The increased risks may be partly attributed to hyperlipidemia, although hypoalbuminemia and hypercoagulable state may be also involved (5).
In adults, risk of CVD can be partly related to traditional risk factors (TRFs), such as advanced age, obesity, and cigarette smoking. These risk factors are not normally present in children with SRNS. However, there are some reports of cardiovascular events in these children. In this regard, Silva et al. reported a case of acute MI in a 7-year-old boy with SRNS (6), and Hopp et al. reported MI in another 7-year-old boy, who had been diagnosed with SRNS 5 years before ischemic heart disease (7).
Assessment of common carotid artery intima-media thickness (A-cIMT) is a relatively reliable and simple measure. In the atherosclerosis risk in communities (ARIC) study, CCA-IMT was compared with all carotid artery segments (A-cIMT), complemented with TRFs and plaque information, in terms of coronary heart disease risk prediction.
With this background in mind, in this pilot study, we aimed to determine the incidence of abnormal carotid intima-media thickening (cIMT) as an early sign of atherosclerosis in a small group of children with SRNS.
2. Methods
We recruited children with primary SRNS who met the following inclusion criteria: (1) age above 5 years; (2) diagnosis of SRNS at least 3 months prior to the study; and (3) renal biopsy with defined underlying histopathology. On the other hand, we excluded children with stage 2 (or above) chronic kidney disease (CKD), as defined by the estimated glomerular filtration rate (eGFR) below 90 mL/min/1.73 M2. Written consents were obtained from the parents.
In all recruited children, cIMT was measured, and blood tests were performed to determine renal function (creatinine and urea levels), serum albumin level, urine albumin/creatinine ratio, lipid profile, C-reactive protein, and complete blood count. The blood samples were routinely processed at the laboratory of great ormond street hospital for children NHS foundation trust.
The body mass index (BMI)-for-age growth chart by the centre for disease control and prevention was used to calculate BMI percentiles (www.cdc.gov). Detailed family history was evaluated in terms of cardiovascular events (before 55 years in males and before 65 years in females), using the expert panel on integrated guidelines for Cardiovascular health and risk reduction. The Schwartz formula was also used to measure eGFR (8).
2.1. Measurement of cIMT
B-mode ultrasound was used to measure cIMT of both common carotid arteries, using a 12-MHz linear-array transducer and a single ultrasound machine (Vivid7, GE healthcare, Horton, Norway). A 15-heartbeat cine-loop recording was obtained for the offline analysis, using the Digital Imaging and Communications in Medicine software. The mean IMT of a segment (0.5 - 1 cm) was measured 1 cm proximal to the carotid bifurcation, using an automated edge detection system on 2 separate images of the right and left common carotid arteries (from true end-diastolic frames), as determined by the minimum vessel diameter. Approximately 120 individual measurements were performed across the artery (1-cm length). The measurements were repeated in 3 different cardiac cycles, and the average of 3 measurements from the right and left arteries was recorded.
2.2. Statistical Analysis
Mean ± standard deviation (SD) was calculated for normally distributed data, and the median (range) was used for the rest of the data. Unpaired 2-tailed t test was used to compare the mean values. Statistical analyses were performed, using GraphPad Prism version 6.0b for Mac OS X (GraphPad Software, La Jolla, California, USA; www.graphpad.com). P value less than 0.05 was considered statistically significant.
In this study, ethical approval was obtained from the Institute of child health of the university college London and the research ethics committee of Great Ormond Street hospital for children NHS foundation trust.
3. Results
A total of 8 children with SRNS were recruited in this study, 5 of whom were female. The mean age of the subjects was 6.9 ± 5.3 years at presentation and 10.8 ± 4.2 years at recruitment. Also, the mean disease duration was 40.9 ± 20.7 months (Table 1). Overall, 4 children were identified as Caucasian, 2 as Indian, and 2 as Pakistani. All children had normal blood pressure (mean systolic blood pressure, 103 ± 7 mmHg; mean diastolic blood pressure, 63 ± 6 mmHg). Also, the mean BMI was 21.8 ± 4.0 g/m2.
| Characteristics | SRNS | Control | P Value |
|---|---|---|---|
| Age, y | 10.8 ± 4.2 | 13.2 ± 4.8 | |
| Gender (F:M) | 5:3 | 19:20 | |
| Age at presentation, y | 6.9 ± 5.3 | ||
| Disease duration, mo | 40.9 ± 20.7 | ||
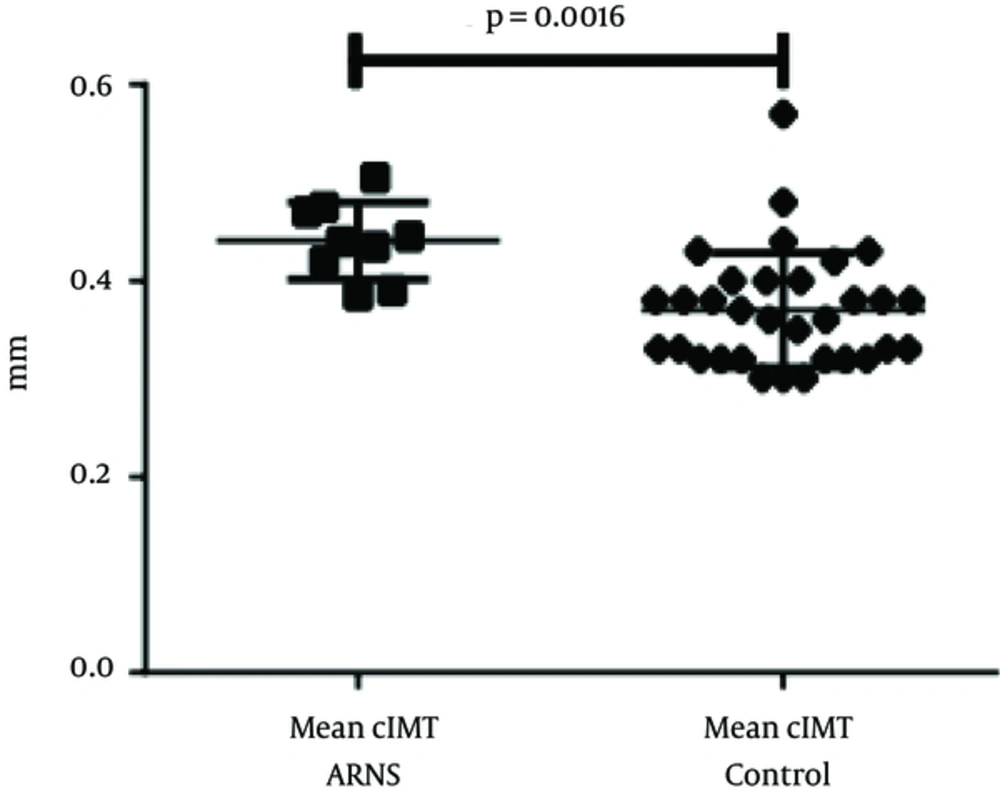
| CIMT, mm | 0.44 ± 0.04 | 0.37 ± 0.59 | 0.0016 |
| Carotid-femoral PWV, m/s (n = 5) | 5.06 ± 0.76 | 5.34 ± 0.97 | 0.5498 |
| Carotid-radial PWV, m/s (n = 9) | 6.97 ± 1.79 |
Abbreviations: CIMT, carotid intima-media thickness; PWV, pulse wave velocity.
Renal biopsy showed focal segmental glomerulosclerosis in 4 cases, minimal change of disease in 2 cases, and membranoproliferative glomerulonephritis in 2 cases. A total of 40 age-matched children were recruited as the controls (9). Table 1 outlines the demographic characteristics of patients and controls. Based on the findings, the mean creatinine level was 45.1 ± 15.0 µmol/L, the mean blood urea level was 4.2 ± 1.8 mmol/L, and the mean eGFR was 120.9 ± 22.9 mL/min/1.73 M2; it should be noted that we excluded children with GFR < 90 mL/min/1.73 M2. All children showed normal CRP, white blood cell count, hemoglobin level, and platelet count.
The analysis of lipid profile showed a mean cholesterol level of 5.4 ± 2.0 mmol/L (normal < 5.2 mmol/L), triglyceride level of 1.74 ± 0.64 mmol/L (normal, 0.45 - 1.71 mmol/L), LDL level of 3.1 ± 2.1 mmol/L (normal < 3.4 mmol/L), very-low-density lipoprotein (VLDL) level of 0.79 ± 0.29 mmol/L (normal, 0.128 - 0.645 mmol/L), and high-density lipoprotein (HDL) level of 1.2 ± 0.3 mmol/L (normal, 0.8 - 1.8 mmol/L).
During the study, 6 children were in the remission stage with a serum albumin level of > 35 gm/L, while the remaining patients (n = 2) had active disease. Only 4 children had normal urine albumin/creatinine ratio (< 30 mg/g), while the remaining patients had significant or nephrotic-range proteinuria with the median of 35.2 (range, 1.8 - 1080) in the whole group.
3.1. cIMT
The mean cIMT was 0.44 ± 0.04 mm for children with SRNS and 0.37 ± 0.06 mm for the controls (Figure 1). Children with SRNS showed a significant increase in cIMT, compared to the controls (0.07 mm; P = 0.0016). However, the values were too small to permit comparison between histological features or treatment types.
4. Discussion
In the present study, we found evidence of early changes in the structure of blood vessels in children with SRNS, as they had thicker CIMT, compared to the controls. This finding is similar to the results reported by Hooman et al. who demonstrated increased cIMT in children with idiopathic nephrotic syndrome (both steroid-sensitive and -resistant), associated with the duration of disease and hypertension (10).
Furthermore, Candan et al. reported changes in cIMT, pulse wave velocity, and left ventricular mass in children with SRNS, which were significantly correlated with proteinuria (11), leading to cardiovascular complications. The main reason is probably multifactorial, including the associated hyperlipidemia (12), hypertension, tendency to thrombosis (13, 14), and use of immunosuppressive medications. SRNS is also associated with increased endothelial markers, such as soluble thrombomodulin, plasminogen activator inhibitor-1, tissue plasminogen activator, and von-Willebrand factor (15), all of which are related to endothelial activation.
Despite achieving remission in the majority of cases during the present research, the study group had an atherogenic lipid profile with high cholesterol level. Nevertheless, the effect of long exposure to disease could persist even after remission, as shown in previous studies (10). We excluded children with stage 2 CKD (or above) in order to avoid the confounding effect of impaired renal function in the cardiovascular system (16).
Overall, use of lipid-lowering agents in children with SRNS is limited, and only few reports are available (17). However, given the reported association between increased VLDL level and subclinical CVD in children with SRNS (10), such treatments could be indicated; in fact, more comprehensive studies are required in this area.
4.1. Conclusions
Children with SRNS showed enhanced cIMT, thus indicating the increased risk of CVD.