1. Background
Dental caries is one of the most common infectious diseases of the oral cavity (1-4) and one of the most common diseases during childhood (5), with several factors affecting its onset and progression (6). Caries result from complex interactions between acid-producing bacteria, fermentable carbohydrates, and host factors (3), and the prevalence of caries is increasing among children and adults in developing countries (3). In spite of the fact that it is highly preventable, it is still considered as the most common chronic disease, worldwide (3).
Saliva can modulate the process of decay as a rich source of host factors, each of which plays a role in preventing dental caries (7, 8). It has been well-documented that saliva secretion and its contents play a significant role in dental health (5, 9). The colonization and removal of oral microorganisms, which may be affected by organic and non-organic salivary compounds (10, 11), activate the inflammation process, and the innate and acquired immune host response (2). Most immune cells, such as neutrophils, lymphocytes, and macrophages secrete numerous pro-inflammatory cytokines, such as IL-6, IL-4, IL-1-β, IL-1- α, IL-10, tumor necrosis factor (TNF-α), and lymphotoxin-alpha (12, 13). The role of TNF-α in host defense and inflammatory responses has been well-documented as a cytokine with capacity of multiple biologic effects that can induce inflammatory cells infiltration and activate phagocytic mechanisms (12, 13). Necrosis factor-alpha (TNF-α) is a pro-inflammatory cytokine first discovered as a protein capable of producing necrosis in mouse-transmissible tumors, and is now known as a cytokine with multiple biologic capacities. This protein affects the growth, differentiation, and function of all cell types and is believed to be an integral part of the interactions of inflammatory and immunological events. This cytokine, along with IL-6, are considered as two key cytokines in the process of acute inflammation and are essential for the development of specific immune responses (14, 15).
Despite the advances in methods for early diagnosis and effective prevention of dental caries, global chronic problems in this regard are still encountered. The increasing trend of its prevalence among children in developed countries has raised concern about public health (9). A careful caries’ risk assessment can identify patients at high risk of decay for therapeutic purposes and improve the efficacy of treatment (16). The decay risk assessment can estimate the risk of decay, such as the number of new cavities or primary lesions at a given time, and the likelihood of a change in the size or activity of decay lesions (17).
The role of saliva and its biological content has been extensively studied to discover its association with dental caries (16), and pro-inflammatory cytokines have been suggested to play a very important role in the immune system. Thus far, limited studies have considered the concentration of pro-inflammatory cytokines in dental caries (18, 19), and it has been shown that TNF-α, IL-6, IL-8, and vascular endothelial growth factor (VEGF) are secreted more in saliva of individuals with active caries (19-21), although there was no relationship between dental caries and the serum and salivary levels of IL-1-β, IL-10, and antagonist IL-1 receptor (18). Also, no study has addressed the relationship between TNF-α levels and age, or compared children’s immune response at different age groups and during adolescence.
2. Objectives
The present study aimed at evaluating the pro-inflammatory cytokine level of TNF-α in saliva and its association with caries in different age groups of children and adolescents.
3. Methods
3.1. Study Population
In this case-control study, 128 children and adolescents (males and females) were divided to four groups of 32, including 32 children aged three to five years, 32 children aged six to eight, 32 children nine to twelve, and 32 adolescents aged thirteen to eighteen years old. Children, who had a positive history of any disease or were under medication were excluded. Samples were selected from schools and kindergartens of Zanjan city after oral examinations by only one person for uniformity of diagnosis. Teeth were examined with an explorer. Teeth with caries existed in at least one site (buccal, lingual, mesial, distal, occlusal or cervical), involving dentin with soft consistency reported as positive. Demographic information and data on dental caries were recorded for all individuals. Children with more than four active caries were included in the case group and children with no caries were defined as the controls. For completing the sample size, a total of 1600 people were screened. The protocol of the study was approved by the Ethics Committee of the Dental School of Zanjan University of Medical Sciences with code ZMUS.REC.1394.307 and the participants were included in the study after obtaining informed consent from their parents.
3.2. Saliva Sample Collection
Before sampling, the participants were asked about their use of any medication during the past two weeks. Also, the participants in the study were banned from eating and drinking for two hours before sampling; then they brushed their teeth (without using toothpaste), and after washing, 5 mL of non-stimulated saliva was spitted in sterile tubes for 15 minutes. All samples were collected from 9 to 12 am to avoid the risk of error in saliva concentration by circadian rhythms. After collecting samples, 1 mL of each sample was centrifuged for five minutes at 12000 rpm.
3.3. Evaluation of Salivary TNF-α Level
Salivary level of TNF-α was measured using a standard kit for salivary Biomarker TNF-α (IBL.Germany.Ref: BE55181), according to the manufacturer’s instructions. The color intensity was calculated by the ELISA reader at an initial wavelength of 450 nm and a reference wavelength of 630 nm. The minimum measurable dose of TNF-α was 2.3 pg/dL.
3.4. Statistical Analysis
The mean and standard deviation of salivary TNF-α level were reported. A general linear model was used for comparison between groups with and without active caries at all age groups. The significance level was considered as P value < 0.05. All data were analyzed by SPSS version 16.0 Chicago, SPSS Inc.
4. Results
A total of 79 children (30 males and 49 females) were studied in two groups with active caries (case group) and without caries (control group). The case group (more than four teeth with active caries) consisted of 45 participants (57%) and the control group (without caries) comprised of 34 participants (43%).
The TNF-α levels were significantly higher in the dental caries group than in the control group (P = 0.001). The mean TNF-α levels in the case group was 35.20 ± 16.23 Pg/mL and in the control group, this was 26.20 ± 6.25 Pg/mL.
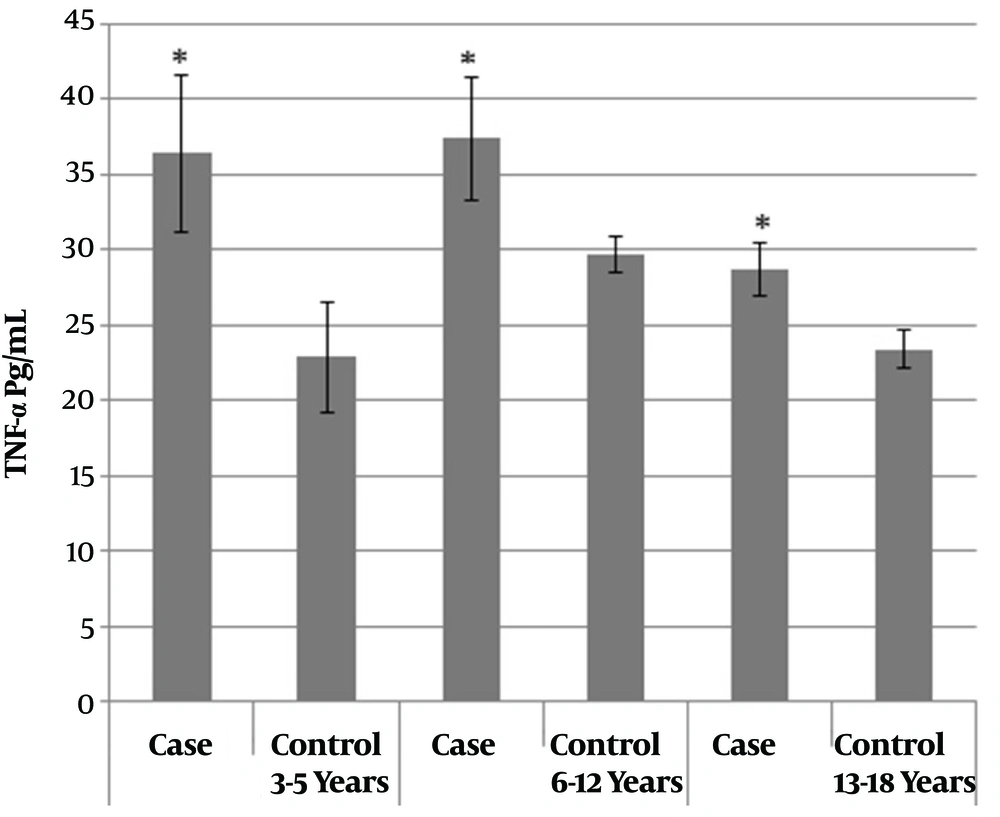
The children were also studied in different age groups. The mean TNF-α level was significantly higher in the age group of six to twelve years than the age group of thirteen to eighteen years (P value = 0.01). The age group of six to twelve years old, at the beginning of the study and at the time of sampling, consisted of two groups of six to nine years (early mixed dentition) and ten to twelve years old (late mixed dentition), unified due to lack of statistical difference. Therefore, the children were divided to three age groups: Three to five (N = 19), six to twelve (N = 34), and thirteen to eighteen years old (N = 26). Mean cytokine levels, irrespective of caries, in the first age subgroup (three to five years) was 29.63 ± 17.08 Pg/mL, in the second subgroup (six to twelve years) was 33.42 ± 14.46 Pg/mL, and in the third subgroup (thirteen to eighteen years) was 26.06 ± 5.88 Pg/mL. The comparison of TNF-α levels between the case and control groups based on age categories is shown in Figure 1. In all age groups, the mean TNF-α level was significantly higher in the case group than in the control group (P value < 0.05). In order to investigate the interaction between the effects of the main study groups, gender, and age groups on mean TNF-α cytokine levels, inter-group effects were evaluated. The intergroup effects (Table 1) showed only statistically significant differences between the two main groups with and without caries (P value = 0.001).
| Source | Df | Sum of Squares | Mean Square | F Value | P Value |
|---|---|---|---|---|---|
| Intercepts | 1 | 3376.71 | 339.701 | 2.12 | 0.030 |
| Main groups | 1 | 1171.02 | 1171.02 | 7.31 | 0.001 |
| Gender | 1 | 1.22 | 1.22 | 0.008 | 0.930 |
| Age groups | 2 | 595.69 | 296.34 | 1.85 | 0.1 |
| Main groups × gender | 1 | 59.25 | 59.25 | 0.37 | 0.545 |
| Main groups × age groups | 2 | 159.35 | 79.67 | 0.49 | 0.610 |
| Gender × age groups | 2 | 104.03 | 52.02 | 0.32 | 0.724 |
| Main groups × gender × age groups | 2 | 424.23 | 212.11 | 1.32 | 0.272 |
The mean cytokine level in males was 30.56 ± 3.76 Pg/mL and in females, this was 31.79 ± 13.54 Pg/mL, which did not show a significant difference (P value = 0.69).
5. Discussion
Despite the advances made in immediate diagnostic methods and effective prevention measures, dental caries still remain one of the most common chronic diseases in the world. The increasing incidence of this disease among children in developing countries is a serious health challenge (22, 23). The present study was conducted to evaluate the hypothesis that there is increased TNF-α levels in the saliva of children with dental caries compared to children without dental caries. This hypothesis was studied in three age groups of children: Three to five (primary dentition), six to twelve (mixed dentition), and thirteen to eighteen years old (permanent dentition). The cytokine levels were also compared among these three age groups.
Cytokines, including TNF-α, played a vital role in immunity and inflammation (24). Cytokines and other factors are useful diagnostic and monitoring tools for the oral cavity, and saliva can be used as a non-invasive diagnostic fluid during initial and advanced stages of the disease to measure biological markers. Cytokines regulate various aspects of the immune response. Although the effect of TNF-α is unknown on the dental caries phenomenon, changes in the cytokines in oral mucosal diseases, such as lichen planus, oral squamous cell carcinoma, recurrent aphthous, premalignant lesions, and periodontal problems, have been shown in previous studies (25, 26). The results revealed that the pre-inflammatory cytokines of TNF-α in children with dental caries were higher in all three age groups, compared to the control group (children without dental caries). These results were consistent with the results of studies conducted by Gornowicz et al. (19) and Sharma et al. (20) that examined the levels of TNF-α, interleukin (IL)-8, and IL-6 cytokines in the saliva of patients with dental caries. In all patients with dental caries, the levels of the cytokines were clearly higher than normal levels. It appears that decay can also activate the inflammatory process in the immune system by a destructive process and eventually the release of TNF-α.
Other studies investigating the relationship between caries and other salivary biomarkers reported increased biomarkers, which is in line with the current study; for instance a study by Gornowicz et al. (27) reported on significant increases in the levels of secretory immunoglobulin A (S-IgA), histantine-5, and lactoproxidase in 18-year-old adolescents with high caries. A study by Zhao et al. (9) showed that the concentration of soluble toll-like receptor 2 (TLR-2) was significantly higher in 6 to 12 year-old children with dental caries than those without dental caries. Ranadheer et al. (28) who studied salivary levels of IgA and its relationship with dental caries in children, showed that salivary levels of soluble IgA were significantly higher in the high caries group than in the group without caries. Ribeiro et al. (21) also reported higher levels of VEGF and IL-6 in children with early childhood caries. Among previous studies, the study of Cogulu et al. (18) found no association between the elevation of salivary and serum levels of IL-1-β, IL-1, and IL-10 receptor antagonist.
According to advanced search in previous studies, which investigated cytokine TNF-α and its relationship with caries, only one study was similar to the present study and the current study was the first to examine TNF-α cytokines in three age groups and their association with these three age groups; no previous study with this methodology was found regarding TNF-α. In this study, the maximum cytokine level of TNF-α was observed in children aged six to twelve years old with dental caries and the increase in TNF-α cytokines was significant in the age group of six to twelve years old compared to the age group of thirteen to eighteen years old. Also, in six- to twelve-year-old children without caries, the mean level of TNF-α (29.64 Pg/mL) was higher than the mean TNF-α levels in adolescents aged thirteen to eighteen years with caries (28.74 Pg/mL).
It is noteworthy to mention that children have mixed dentition in the age group of six to twelve years old, and the presence of mobile primary teeth as well as their eruption in the mouth cause an inflammatory process that can justify the increase of pro-inflammatory cytokines as a confounding factor. It has been previously mentioned that the age group of six to twelve years old, at the beginning of the study and at the time of sampling, consisted of two groups of six to nine (early mixed dentition) and ten to twelve years old (late mixed dentition) for a smaller range of age and for measuring the age effect more accurately yet the researchers unified these two groups due to the lack of statistical difference.
In addition, as children are at their puberty at the end of the age range of six to twelve years, the relationship between puberty and the immune system should be outlined; the immune response cytokine and interleukin pathways can be involved in the regulation of the reproductive system, due to the induction of secretion of gonadotropins, such as follicle stimulating hormone and luteinizing hormone. It seems that the relationship between the endocrine and immune system is decisive in expressing interleukin in puberty (29). The reported results related to the production of inflammatory cytokines and their age variations are conflicting and different inflammatory cytokines have been reported to increase in different age groups (30, 31).
In a study by Kamma, which examined the levels of IL-1β, IL-4, and IL-8 cytokines in gingival fluid in adolescents (14 to 16 years old) and young people (25 to 35 years old), a different response to local immune system was reported without significant differences in dental plaque of the two age groups, which led to the hypothesis that change in expression of cytokines may be related to age (32). Thus, the gingivitis rate was determined at all ages of childhood. Gingivitis with the same plaque level gradually increases from early childhood to early teenage years, and then this trend is stopped and fixed in the second decade of life (32). This theory is consistent with the increase of TNF-α in mixed dentition, compared to the other two groups (children with primary and permanent dentition), according to the present study. Increased gingivitis during puberty compared with infancy, in addition to local factors, such as increased number of plaque-affected sites, wider plaque volume, and inflammatory changes associated with tooth decay or tooth loosening may be influenced by systemic factors (such as hormonal changes related to puberty), as it is believed that increased sex hormones during puberty have a transient effect on gingivitis (33), and human gingiva can metabolize sex hormones. Vittek showed a direct relationship between progesterone and inflammation around the tooth, as inflammation increased in patients with former gingivitis (34). These effects can be seen as one of the changes during puberty, as the circulation of sex hormones increases in the body. These studies are in accordance with the results of the present study and can justify the difference and increase in TNF-α cytokines in the age group of six to twelve years old compared to that of three to five years old.
5.1. Conclusion
The results of this study indicated that decay plays an important role in increasing cytokine TNF-α in non-stimulatory saliva, yet since there is no confirming evidence of the direct effect of age on immune function, more studies with longer follow-ups are required in all three age groups to confirm the role of TNF-α as a valuable diagnostic biomarker, as well as its relationship with age. Future studies can also consider other biomarkers and other age groups, including middle aged and old aged groups.