1. Background
The pervasive developmental disorders are pediatric problems which result in major impacts in lives of children (1). Autism is one of these disorders initially introduced by Kanner’s in 1943 among children who were disabled in social communication (1, 2). The typical autism prevalence among five-year-olds in Iran is reported 6.3 per 10,000 children (3). Ghanizadeh estimated the prevalence of pervasive developmental disorders such as ASD (autism spectrum disorders) 190 per 10,000 in Shiraz (central part of Iran) (4). According to DSM-V-TR (The Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision, American Psychiatric Association) autism is the most common type of pervasive developmental disorder (2). These children usually have verbal problems, weak eye contact, anxiety, fear of changes, and motor problems; all leading to learning disorders (5-9). They are dependent on non-living things and images. Hence treatment of these children is crucial. The purpose of treatment is to help children with autism to acquire functional skills in routine life and to control behaviors that are disruptive. The current indications of Cerebrolysin in practice are as follows: it is FDA-approved in USA as a novel treatment of alzheimer in adults. Cerebrolysin is used as an off-label treatment in management of spasticity in children with cerebral palsy and traumatic brain injury aged up to 18 years old. It Improves cognitive status in children with Rett syndrome, autism and attention deficit hyperactivity disorder (ADHD) (10).
Different classes of pharmacological agents are effective in control of behavioral symptoms of ASD such as tricyclic antidepressants (imipramine), neurotransmitter reuptake inhibitors (fluoxetine), atypical antipsychotics (clozapine), anticonvulsants (lamotrigine), and acetylcholinesterase inhibitors (rivastigmine), etc. (11).
Cerebrolysin is a neuropeptide preparation. It has neurotrophic and recovery effects after various insults to brain. The preclinical application of cerebrolysin promises different capabilities and its multi-target effects (12). The mechanism of Cerebrolysin is to augment the quantity of blood-brain-barely glucose transporter GLUT-1 (glucose transporter-1) in vitro and in vivo (13). Cerebrolysin is able to reduce the neurotoxicity, inhibit the free radical production, activate the microglia, trigger the neurotrophic activity, promote the neuronal budding, enhance the cellular life expansion, and improve the neurogenesis after cerebral stroke (14). It improved language development and com¬munication, and notably, symbolic behavior in infants with severe perinatal brain insult (15). Crebrolysin effect has also been confirmed in acute ischemic stroke treatment, autism and vascular dementia, and other neurodegenerative disorders (16). Preclinical and clinical data on Cerebrolysin support its efficiency in mild to severe Alzheimer’s disease as a suitable treatment option (17). Cerebrolysin is generally safe and well-tolerated by children. Adverse drug reactions are usually mild and may include: Flue-like syndrome, confusion, anxiety, agitation, diarrhea, and vomiting (15, 18-20). Autism spectrum disorders have disabling effects on different aspects of parents’ life. Therefore, minor improvement in cognitive, repetitive behaviors and verbal abilities of children with autism could be greatly helpful to improve quality of life of their parents. Although no special treatment is certified for autism spectrum disorders, various treatment trials were carried out in different parts of the world and cerebrolysin has been one of these drugs. Cerebrolysin in autism has not been studied yet in our country, thus evaluation of cerebrolysin effect in partial improvement of autistic children could be promising.
2. Objectives
In this study the safety and efficiency of cerebrolysin on behavioral nonverbal and verbal development was determined in children with autism referred to the neurology clinic of Children’ Medical Center in Tehran, since September 2014 to March 2015.
3. Methods
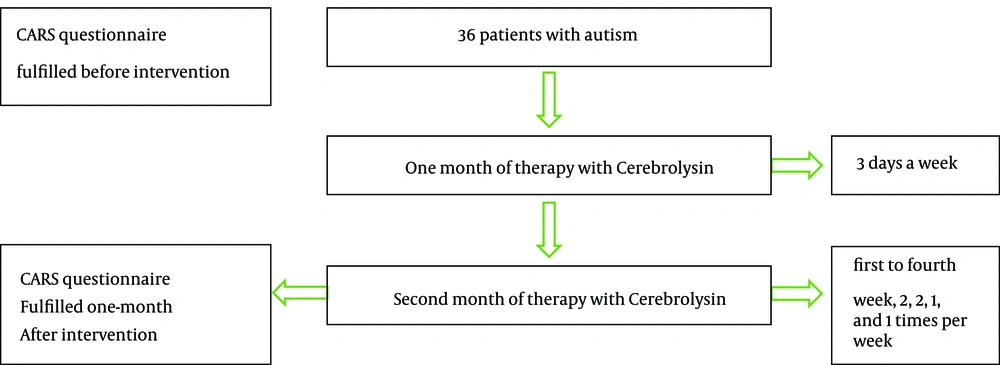
In an interventional quasi-experimental study, 40 consecutive children were enrolled aged from 3 to 10 years with the diagnosis of autism (confirmed by DSM-V). The sample size was determined around 40 cases based on statistics of similar studies and the number of patients that were referred to neurology clinic. From total 40 children, four patients were excluded from the study due to adverse drug reactions including agitation and unusual irritability related to fear of interamuscular injection. This study was accredited by Ethical Committee of Tehran University of Medical Sciences. Helsinki Declaration was respected across the study and the informed consent form was signed by the parents. Neither of the parents accepted to enroll their children in control group with placebo intervention. Therefore a control group with placebo intervention to compare the results with main group was not available. The inclusion criteria were determined for all referred children aged 3 to 10 years with the diagnosis of autism who had responded partially to other medications like anti-psychotic drugs by the attending neurologist. The exclusion criteria included the consumption of any drug or medical intervention in recent three months, uncontrolled seizures, kidney failure, past history of cerebral hemorrhage and the development of major drug adverse effects. The previous treatments were similar in all patients including only antipsychotic drugs. Cerebrolysin was added to previous therapies, for example in patients who were receiving risperidone, which was continued during our study. A limitation in this study was that other treatment modalities including rehabilitation, occupational therapy and speech therapy were received by patients simultaneously. So, they could have confounded the results.
Drug was administered 0.1 cc/Kg via intramuscular route (15, 19), three days a week in the first month. In the second month, first to fourth week the patients received 2, 2, 1, and 1 injection per week (Figure 1). Each patient received totally 18 intramuscular injections. In similar studies, totally 10 injections were given, but we decided to perform 18 injections in order to improve the drug efficacy. Also, adverse drug reactions were assessed at injection periods. No adverse drug reaction was observed during this study except injection phobia and pain at injection site. Most common adverse events repotrd in the literature include vertigo, agitation and feeling hot. In the controlled clinical trials, the incidence of adverse events was similar in Cerebrolysin- and placebo-treated groups. Cerebrolysin seems to be safe when used in combination with recombinant tissue-type plasminogen activator or cholinesterase inhibitors such as donepezil or rivastigmine. Cerebrolysin was not associated with major changes in vital signs or laboratory parameters (21).
The safety and efficiency of Cerebrolysin on behavioral, verbal and nonverbal development among autistic children was determined by CARS questionnaire filled before and one month after complete intervention. Paired sample t-test was used to compare the results before and after intervention. The validity and reliability of CARS questionnaire was confirmed after its translation into Persian (22).
The Childhood Autism Rating Scale (CARS) is a 15-item observation-based behavior rating scale widely used for screening and diagnosis of autism. The test has to be completed by the clinician during the study. It is used to differentiate autistic children from those with developmental delays without characteristics observed in autism spectrum disorder. Each item is rated 1 to 4 (1, 1.5, 2...4). The score of one stands for normal limits for that age, and the score of four reflects severely abnormal child for that age. Summing the ratings on all 15 items ranges from 15 to 60. The scale is used to subjectively rate fifteen items including relationship to people, imitation, listening response, visual response, adaptation to change, taste-smell-touch response and use, activity level, level and consistency of intellectual response, emotional response, body, object use, fear and nervousness, non-verbal communication and general impressions. The CARS has three types; standard version rating booklet (ST), high-functioning version rating booklet (HF) and questionnaire for parents or caregivers (QPC) (23).
Data analysis was conducted on 36 patients using SPSS software, version 20.0 (Statistical Procedures for Social Sciences; Chicago, Illinois, USA). Paired sample t-test was used in this study. P values less than 0.05 were considered valuable.
3.1. Primary and Secondary Outcomes
The primary outcome of this study was improvement in CARS scores in language and secondary outcome was change in cognitive and behavioral criteria 3 months after initial prescription of Cerebrolysin.
4. Results
Totally 22 male and 14 female patients with mean age of 5.96 ± 2.15 years (ranging from 3 to 10 years) were enrolled in the study. The mean paternal and maternal age was 38.1 ± 7.2 and 34.7 ± 7.2 years, respectively. The positive family history for autism was present in one case. All children but two were born at term and had normal birth weight. Fifteen children (41.7%) had abnormal motor development. Prenatal and perinatal problems were seen in six children (16.7%). Parental consanguinity was detected in nine (25%) cases.
All evaluated items except level and consistency of intellectual response had favorable reduction in scores of CARS questionnaire (P = 0.001). The total score was decreased from 40.6 to 36.1, showing 11.1% improvement. The results are shown in Table 1.
| CARS Items | Before | After | P Value |
|---|---|---|---|
| Relating to people | 2.701 ± 0.6148 | 2.132 ± 0.5123 | 0.000 |
| Imitation | 2.799 ± 0.6319 | 2.493 ± 0.5991 | 0.001 |
| Emotional response | 2.639 ± 0.4944 | 2.306 ± 0.4396 | 0.000 |
| Body use | 2.563 ± 0.4070 | 2.299 ± 0.4505 | 0.001 |
| Object use | 2.861 ± 0.3456 | 2.576 ± 0.3771 | 0.001 |
| Adaptation to changes | 2.583 ± 0.5312 | 2.257 ± 0.5017 | 0.001 |
| Visual response | 2.590 ± 0.5737 | 2.271 ± 0.5049 | 0.001 |
| Listening response | 2.368 ± 0.5716 | 2.188 ± 0.5586 | 0.000 |
| Taste-smell-touch response and use | 2.750 ± 0.4053 | 2.403 ± 0.4403 | 0.001 |
| Fear and nervousness | 3.549 ± 0.4505 | 3.125 ± 0.6505 | 0.001 |
| Verbal communication | 2.368 ± 0.2638 | 1.944 ± 0.2164 | 0.001 |
| Non-verbal communication | 2.701 ± 0.4622 | 2.403 ± 0.4237 | 0.001 |
| Level and consistency of intellectual response | 2.512 ± 0.2835 | 2.509 ± 0.2447 | 1.000 |
| Activity level | 3.063 ± 0.5154 | 2.896 ± 0.4527 | 0.001 |
| General impressions | 2.590 ± 0.4711 | 2.326 ± 0.4583 | 0.001 |
| Total | 40.611 ± 4.6811 | 36.099 ± 4.2823 | 0.001 |
aValues are expressed as mean ± SD.
5. Discussion
Cerebrolysin efficiency has been evaluated in cerebral stroke, Alzheimer’s disease, and other neurological conditions in many clinical trials conducted around the world. Since the early 1970’s, Cerebrolysin has been studied in numerous double-blind placebo controlled trials. Cognition impairment, mood changes, sustained improvement in memory, slowing down of progressive memory loss, and motor and sensory improvements in patients with stroke and neurodegenerative diseases were reported with Cerebrolysin treatment (24). In the United States, Cerebrolysin has been approved for treatment of Alzheimer’s disease. Cerebrolysin is produced by Ebewe pharmaceutical. Since 1973, over 176 articles have been issued regarding Cerebrolysin benefits on different neurological diseases (25).
In a study by Chutko et al. the efficiency of Cerebrolysin in treatment of autism spectrum disorder was evaluated by observing clinical and neurophysiological changes in affected children. In this study, 43 autism children aged 4 to 6 years were assessed by means of quantitative scale of autism severity according to CARS questionnaire. The patients were compared based on clinical and electroencephalographic examination and classified in to exogenous (organic) and endogenous variants. The lower scores of CARS and higher cerebral cortex functional immaturity was detected in endogenous autism. Totally 27 children (62.8%) showed improvement in signs. Improvement was considered in 13 patients (56.5%) with endogenous autism and in 14 children (70%) with organic autism (26).
In another study by Chen et al. Cerebrolysin was reported as an effective agent in improvement of cognitive function in patients with mild traumatic brain injury during 3 months after injury, significantly drawing ability and long-term memory (27). Cerebrolysin treatment in girls with Rett syndrome performed by Gorbachevskaya et al. showed improvement in attention level, motor function, behavior and nonverbal social communication and normalization of Electroencephalogram (28).
Similarly in a study by Cuevas et al. the effect of Cerebrolysin (daily injection of 2.5 mL/Kg i.p. during 15 days) was studied on a rat model with autism. Total 43 male and 51 pregnant female rats were injected with valproic acid (600 mg/Kg) at the embryonic day 12.5, in order to demonstrate behavioral and synaptic impairment.
In another prospective clinical study by Radzivil and Bashina (29) 25 patients with autism aged from 3 to 8 years received two courses of injections (15 intramuscular injections, 1.0 mL every other day) with 2 months interval and basic antipsychotic therapy (typical neuroleptics). After the 1st Cerebrolysin course, significant improvement was detected in 38% patients. Improvement was reported in approximately 50% after the second course of injections and 71% during follow-up (180th day) which was lower than 90% improvement compared to the present study. The autism severity based on the CARS scale decreased from the day 0 to the day 180, from 37.7 to 32.6 scores, respectively. The patients demonstrated a considerable decline in mental retardation, a great improvement in attention during task performing, receptive and expressive speech, fine motor function, cognitive activity, cognitive performance and perception (30).
Krasnoperova et al. evaluated nineteen children aged 2 - 8 years with childhood autism and 8 with Asperger’s syndrome. Cerebrolysin was prescribed for treatment in inpatient clinic. All the patients received 10 injections (intramuscularly) of 0.1 mL Cerebrolysin, daily during 5 days. Cerebrolysin resulted in improvement of cognitive functions (expressive and receptive speech, fine motoring, and playing). All patients with Asperger’s syndrome and 89% patients with childhood autism revealed improvement. No therapeutic adverse effects were seen (20) as in our study. The current study was carried out on a greater sample size compared to Radzivil et al. (29) and Krasnoperova et al. (20) study.
The main difficulty in performance of this research was that neither of the parents accepted to enroll their children in control group with placebo intervention. Another difficulty was lack of previous studies in the research area regarding safety and efficiency of Cerebrolysin. Literature findings are the foundation for the researcher to achieve research objectives. There were limited clinical trials to assess Cerebrolysin efficacy and safety in autism children prospectively.
The most significant advantage of this study was the first administration of a new medicine in Iran in order to improve the cognition, nonverbal and verbal aspects of children with Autism spectrum disorders.
Despite the efficiency of Cerebrolysin observed in this study, its partial use may be limited by the large number of intramuscular injections, i.e. nine per month. Sustained release formulation may alleviate this limitation if this therapy proves to be beneficial in future studies.
5.1. Conclusions
In the present study the effect of Cerebrolysin on behavioral, nonverbal and verbal development in children with autism was assessed and all evaluated items except level and consistency of intellectual response showed favorable reduction. Total CARS score demonstrated more than 10% improvement. Also, ninety percent of children showed improvement. This partial response was determined as an acceptable result in children with autism.
According to the obtained results, the safety and feasibility of Cerebrolysin administration could be concluded in children with autism. It is effective as an add-on therapy in behavioral, nonverbal and verbal development of autistic children. Moreover, future double-blind placebo control trials can approve this result. All in all, this was a preliminary study, so performing further studies with control group, larger sample size, multicenteric sampling, in comparison with other medical and non-medical treatments is suggested.