1. Introduction
Functional constipation (FC) in children often requires medical treatments, including pharmacotherapy. Lubiprostone is derived from prostaglandin E1 and activates type 2 chloride channels in intestinal epithelial cells to increase the secretion of chloride-rich fluid (1). This laxative is considered as a useful drug for FC in adults, and is described in the adult guidelines for FC (2, 3). The Guideline of Pediatric Chronic Functional Constipation Treatment, which was published during November 2013 in Japan (4), recommends lubiprostone for pediatric FC. However, this laxative has never been used in pediatric medicine in Japan because there is no clinical usage experience in children. No pediatric study on lubiprostone has been reported in Japan. Moreover, few studies on lubiprostone administered for FC in children have been published worldwide (5).
This case report describes the first Japanese experience of lubiprostone for intractable pediatric FC, which was not successfully treated using other applicable laxatives. This study describes the empirical measures taken against an acute adverse event (AAE), lubiprostone-induced nausea.
1.1. Patients
This was a single-center, retrospective study carried out at the Division of Pediatrics in Tohoku Medical and Pharmaceutical University. Thirty-three patients, who conformed to Rome III FC criteria (4), including 14 males with median age of 4.1 years, visited the center of the current research from April 2016 to March 2018. The administration of lubiprostone had two limitations at the current research center. The first qualification was that daily dose per weight was within the upper limit, 1.33 µg/kg, according to the literature (5). The second one was that all previously administered laxatives were ineffective. Treatments were estimated as effective when the patients did not meet Rome III FC criteria for more than four weeks (6).
Six patients were suitable for the qualifications. The patients’ backgrounds are shown in Table 1. Pre-administered drugs before lubiprostone were carmellose sodium (CS: 1 case, 0.017 g/kg/day), mosapride citrate (MC: 3 cases, 0.3 mg/kg/day), magnesium oxide (MO: 6 casess, 0.06 g/kg/day), probiotics (3 cases), and sodium picosulfate (SP: 2 cases, 0.3 mg/kg/day). Emergent fecal impaction was recognized in patients one and two. There was no contraindication for pediatric lubiprostone administration, based on the instruction manual and the previous literature (5). The researchers obtained an informed consent for all patients from patients and their caregivers. Patients were administered lubiprostone capsules (24 µg) with approved doses of once per day for patients under 11 years old and twice per day for patients aged 12 years or more. Permission for pediatric lubiprostone usage in these emergent cases was obtained from the committee of pharmaceutical affairs of the current research center because there were no pharmaceutical alternatives. Two of the six patients, patients 1 and 2, received lubiprostone twice, at least 14 months apart.
The results are shown in the Table 1. The patients in all eight administrations showed improvement of defecation (100%), fecal frequency (FF) and Bristol stool scale (BSS). Six administrations of lubiprostone, including the second administrations to patients 1 and 2, were completed without AAEs. Five patients (83.3%) achieved complete remission, laxative-free status for more than four weeks (6), within four months. However, on the first administration of lubiprostone, patients 1 and 2 self-interrupted the administration after dis-impaction, because of nausea and vomiting. Simultaneously administered drugs with lubiprostone were MC, MO, probiotics, SP, and trimebutine maleate (TM; case 1 and 2, 5.8 mg/kg/day). Furthermore, TM was not used during the first administration of lubiprostone in patients 1 and 2.
| Case | Age/Gender | Height/ Weight (cm/kg) | Pre-Lubiprostone SF/BSS | Lubiprostone Capsule Frequency (Per Day) | Simultaneous Administration | Duration | Post-Lubiprostone SF/BSS | Outcome | Lubiprostone (Dose/Time in µg/kg) |
|---|---|---|---|---|---|---|---|---|---|
| 1-First | 8 y 2 m/F | 123.7/22.6 | 1/1 | 1 | 6 daysb | 7/4 | post-treatmentc | 1.06 | |
| 1-Second | 8 y 11 m/F | 129.7/26.6 | 0/1 | 1 | TM | 3 weeks | 7/5 | post-treatmentc | 0.90 |
| 2-First | 8 y 10 m/F | 129.4/35.0 | 0/1 | 1 | MC, MO, SP, Pro | 4 daysb | 7/4 | post-treatmentc | 0.69 |
| 2-Second | 10 y 0 m/F | 140.4/41.4 | 1/2 | 1 | MO, TM | 15 weeksd | 7/5 | completee | 0.58 |
| 3 | 10 y 5 m/M | 134.1/39.9 | 2/1 | 1 | MC, MO, Pro | 13 weeksd | 7/4 | completee | 0.60 |
| 4 | 12 y 11 m/M | 174.8/59.2 | 2/1 | 2 | 2 weeks | 7/4 | completee | 0.41 | |
| 5 | 13 y 1 m/M | 172.6/59.3 | 1/1 | 2 | 6 weeks | 7/4 | completee | 0.40 | |
| 6 | 13 y 11 m/F | 150.3/62.5 | 2/1 | 2 | 4 weeks | 7/4 | completee | 0.38 |
Abbreviations: BSS, Bristol Stool Scale; CS, carmellose sodium; F, female; M, male; m, months; MC, mosapride citrate; MO, magnesium oxide; Pro, probiotics; SF, stool frequency per week; SP, sodium picosulfate; TM, trimebutine maleate; y, years.
aThe content of a lubiprostone capsule was 24 µg.
bThe reason for self-interruption was unbearable nausea in patients 1 and 2.
cPost-treatment means a change to other laxatives.
dAfter the administration of lubiprostone once per day for 4 weeks, an interval was created every other day.
eComplete means good defecation without laxatives.
2. Case Presentation
2.1. Case 1 Presentation
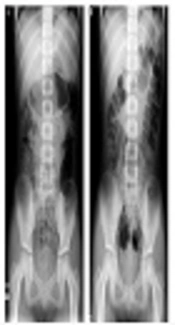
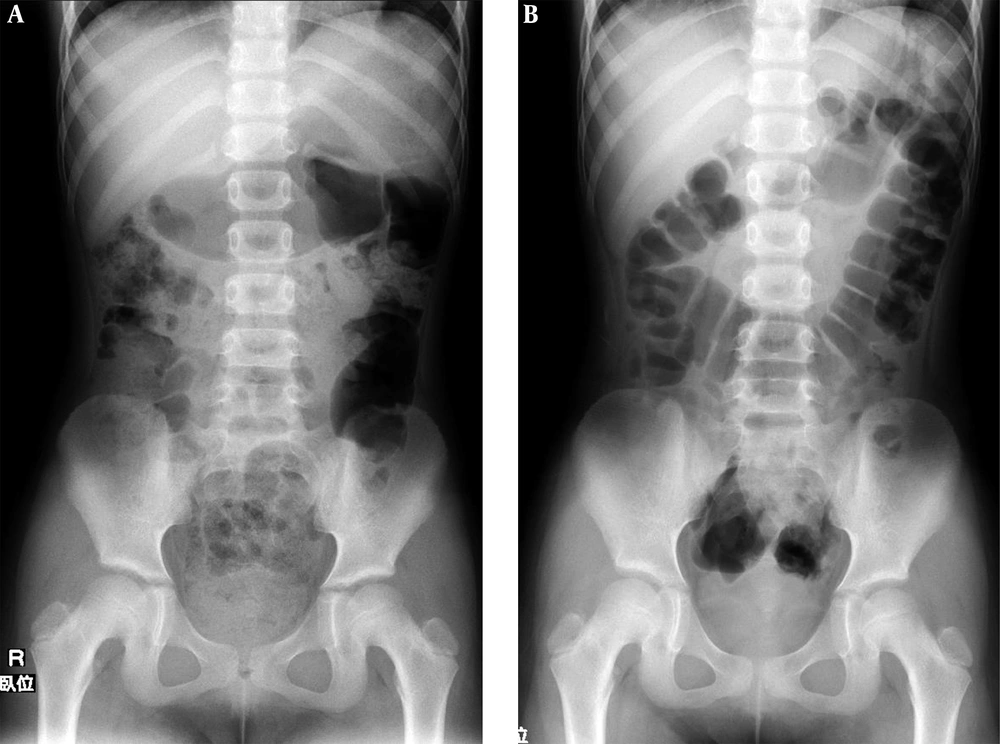
This study describes the second administration of lubiprostone in patient 1. The patient was an eight-year-old female, who self-interrupted lubiprostone because of nausea and vomiting in the previous 15 months. She had received other applicable administrations of CS, MC, MO, and probiotics for nine months. She had fecal impaction, despite good adherence (Figure 1A). The patient and her caregivers agreed to the second administration of lubiprostone, provided there was simultaneous administration of TM. This gastrointestinal motility-regulating agent had been used empirically for lubiprostone-induced nausea in adults at the current center. One 24-µg lubiprostone capsule was administered once a day (0.90 µg/kg/time). After two days, disimpaction was performed without any AAEs, including nausea (Figure 1B). Lubiprostone was administered for three weeks. The administration of MC, MO, and probiotics was continued as maintenance therapy for six months post-treatment.
2.2. Case 2 Presentation
Case 2, an 8-year-old female, followed a similar process such as case 1. After 14 months from the self-interruption, the second 24-µg lubiprostone capsule in addition to MO and TM was administered once per day (0.58 mg/kg/time) for 4 weeks (5). She showed improvement of BSS, yet FF was at a standstill (3 days/week). As maintenance therapy, lubiprostone administration once every other day with MO and TM was performed for 11 weeks. After the improvement of FF (7 days/week), the administration of all drugs was stopped. No recurrence was recognized for two months post-treatment.
3. Discussion
Lubiprostone had excellent efficacy in a short period (within 15 weeks) for pediatric FC, with only one AAE, lubiprostone-induced nausea. Many studies have shown that lubiprostone is effective for FC in adults (1-3). However, pediatric administration of lubiprostone has only been reported in one prospective, multicenter, open-label study (5). The mentioned study reported that lubiprostone administration for four weeks was fast-acting (within one week), and the main effects and AAEs, including nausea were dose dependent. In the literature (1, 3, 5), other AAEs were diarrhea, abdominal pain, and headache. These AAEs were not recognized in all cases at the current center.
Lubiprostone-induced nausea often results in self-interruption of administration, even in adults. The AAE rate, which was 25%, was similar to that in adult studies (2). The detailed mechanism of nausea has not yet been elucidated. In cases of nausea, a reduction in lubiprostone dose is recommended as the first step. As the second step, a gastrointestinal motility-regulating agent should be selected (7). The researchers selected TM because of successful experiences in adults at the research center. However, there is no evidence in the literature supporting the use of TM in cases of lubiprostone-induced nausea.
Because lubiprostone-induced nausea is dose dependent, the researchers analyzed the relevance to nausea of the lubiprostone-dose per weight (kg). There was no evident trend regarding nausea and the daily dose per weight. However, a lubiprostone dose greater than 0.69 µg/kg/time induced nausea when administered without TM for the first time in patients one and two. Trimebutine Maleate inhibited nausea, despite a high dose of lubiprostone (0.90 μg/kg/time) during the second administration of lubiprostone in patient 1.
This case report had some limitations. The first limitation was the available dosage form of lubiprostone in Japan. Only one form of lubiprostone (24-µg capsules) was available as of July 2018. The resulting difficult dosage adjustments in accordance with physical constitution or age can result in lubiprostone-induced nausea. This problem will be ameliorated when lower dosage capsules become available in the near future. The second limitation is the lack of knowledge about the optimal lubiprostone administration period. Lubiprostone administration was performed for periods up to 15 weeks without AAEs, except for nausea, in the patients. No study has reported the optimal administration period of lubiprostone from the perspectives of efficacy, AAEs, and chronic adverse events, even in adults. These limitations should be resolved as experience with additional cases allows statistical analysis to be performed.
3.1. Conclusions
The researchers recommend that lubiprostone administration should initially be less than 0.69 µg/kg/time. When a higher dose is required, gastrointestinal motility-regulating agents, such as TM, should be used concurrently.