1. Background
In patients with dilated cardiomyopathy (DCMP), the impairment of cardiac performance is generally associated with impaired left ventricular (LV) relaxation and diastolic and systolic wall motion abnormalities (1-3). For patients who are severely symptomatic even under maximal pharmacological therapy, heart transplantation is the treatment of choice. However, pediatric heart transplantation is extremely limited because of the lack of donors and high costs in Asian countries (4). Thus, other non-transplantional surgical procedures such as mitral valve repair or left ventricular volume reduction surgery (LVVRS) including partial left ventriculectomy (PLV) have been the only options for pediatric patients with severely depressed LV function caused by DCMP to improve the long-term survival and quality of life (3). It was popularly performed instead of heart transplantation in the mid-1990s because 6-month survival rates were similar to those of heart transplantation at an early stage (5, 6). However, later reports on the early and late survival outcomes are controversial (3, 5-10).
2. Objectives
The first aim of the present study was to report the early and mid-term outcomes of less invasive trans-apical LVVRS performed through a small incision on the LV apex at our institution and the second aim was to carry out an observational analysis of prognostic factors related to early and late death after LVVRS for pediatric patients with end-stage idiopathic DCMP.
3. Methods
The Institutional Review Board of Sungkyunkwan University Health System approved this retrospective study (2017-11-016). We reviewed the medical records of patients who underwent LVVRS for idiopathic DCMP at the Samsung Medical Center between March 1997 and February 2014. The candidates for LVVRS included patients who had New York Heart Association (NYHA) functional class III or IV end-stage heart failure refractory to maximal pharmacological therapy for at least two months who could not wait for heart transplantation and did not have a mechanical assist device available. We excluded secondary dilated cardiomyopathy such as post-myocarditis, drug-induced, tachycardia-induced, and systemic hypertension-associated cardiomyopathy. Patients with previous chemotherapy or radiotherapy were also excluded. Patients’ characteristics, past medical history, grade of NYHA functional state before and after LVVRS, type of LVVRS, pre- and postoperative echocardiographic findings and cardiac catheterization assessments, and early and late postoperative mortality were reviewed. We defined the early failure of LVVRS as death or heart transplantation within two months after LVVRS. We used IBM SPSS for Windows (Version 21.0, Chicago, IL, USA) for statistical analysis. All parameters were expressed as means ± standard deviation or numbers, as appropriate. Kaplan-Meier curve analysis was used to assess the mortality rate, and the values of P < 0.005 were considered statistically significant. Fisher’s Exact test and the Mann-Whitney test were used to assess the differences between the failed group and the surviving group after LVVRS. The Wilcoxon Signed Rank test was used to assess changes in echocardiographic findings before and after LVVRS.
4. Results
In total, 12 patients with idiopathic DCMP were registered during the study period. We excluded two patients because they had only mitral valve repair surgery. Therefore, 10 patients with idiopathic DCMP underwent operations to reconstruct the shape and volume of the LV cavity. There were five males and five females. The mean age at the diagnosis of idiopathic DCMP was 63.10 ± 44.39 (median 50, range 5.00 - 147.00) months, and the mean age at the time of LVVRS was 83.30 ± 68.80 (median 63.5, range 14.00 - 210.00) months. The mean interval from the diagnosis to LVVRS was 20.30 ± 35.34 (median 4, range 1.00 - 114.00) months. When comparing the LVVRS group and the total idiopathic DCMP group, there was a statistically lower fractional shortening in echocardiographic parameters and a higher level of the N-terminal fragment of the prohormone brain-type natriuretic peptide (NT-proBNP) in the LVVRS group when they had been diagnosed (Table 1).
| At Diagnosis | LV Volume Reduction Surgery (N = 10) | Total Idiopathic DCMP (N = 12), Mean ± SD | |
|---|---|---|---|
| Mean ± SD | Min - Max | ||
| Age (months) | 63.10 ± 44.39 | 5.00 - 147.00 | 58.61 ± 71.68 |
| Body weight (kg) | 18.81 ± 11.51 | 6.20 - 40.40 | 20.31 ± 19.38 |
| Height (cm) | 111.38 ± 28.10 | 68.80 - 157.00 | 104.18 ± 40.80 |
| EF by M-mode (%) | 22.92 ± 9.53 | 15.00 - 44.00 | 27.70 ± 10.40 |
| EF by Simpson (%) | 21.75 ± 3.41 | 18.70 - 26.00 | 27.51 ± 10.87 |
| FS (%) | 11.03 ± 5.35a | 6.70 - 22.00 | 13.68 ± 5.52 |
| LVEDD/BSA (mm/m2) | 88.78 ± 26.03 | 54.92 - 135.15 | 89.83 ± 33.06 |
| LVESD/BSA (mm/m2) | 79.73 ± 25.18 | 49.24 - 126.00 | 77.96 ± 29.34 |
| NT-proBNP (pg/mL) | 23,904.50 ± 11,658.07a | 15,661.0 - 32,148.0 | 11,937.21 ± 12,063.71 |
Clinical, Echocardiographic, and Laboratory Characteristics of Patients
4.1. Surgical Technique
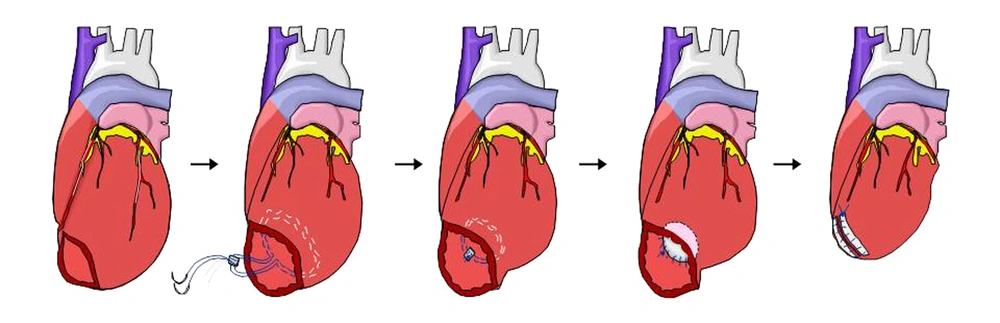
The modified Dor procedure, a patch reduction of the LV through a small apical incision without muscle resection (trans-apical LVVRS), was performed in nine patients, and the Batista procedure was performed in one remaining patient. In the trans-apical LVVRS, after inducing cardioplegic arrest with antegrade intermittent cold blood perfusion, the LV was opened through a 2 - 3-cm long linear incision on the thin apical area, identified using digital palpation. Two circular purse-string stitches with 2 - 0 or 3 - 0 monofilament sutures reinforced with Teflon pledgets were made along the bases of the papillary muscles. If the myocardium was too thin, the surgeon was careful not to damage the epicardial coronary arteries. The circular sutures were passed through the previous Teflon pledget and tied down to constrict the apical portion of the LV. The final diameters of the constricting necks thus constructed were between 1.5 and 2.0 cm, approximately one-third of the original diameters. An appropriate size piece of bovine pericardium (Periguard, Biovascular Inc, Saint Paul, USA) was applied along the circular suture lines using 3 - 0 or 4 - 0 monofilament continuous sutures. The apical incision was closed using double-layer 3 - 0 monofilament sutures reinforced with the bovine pericardial strip (Figure 1).
4.2. Mid-Term Outcomes
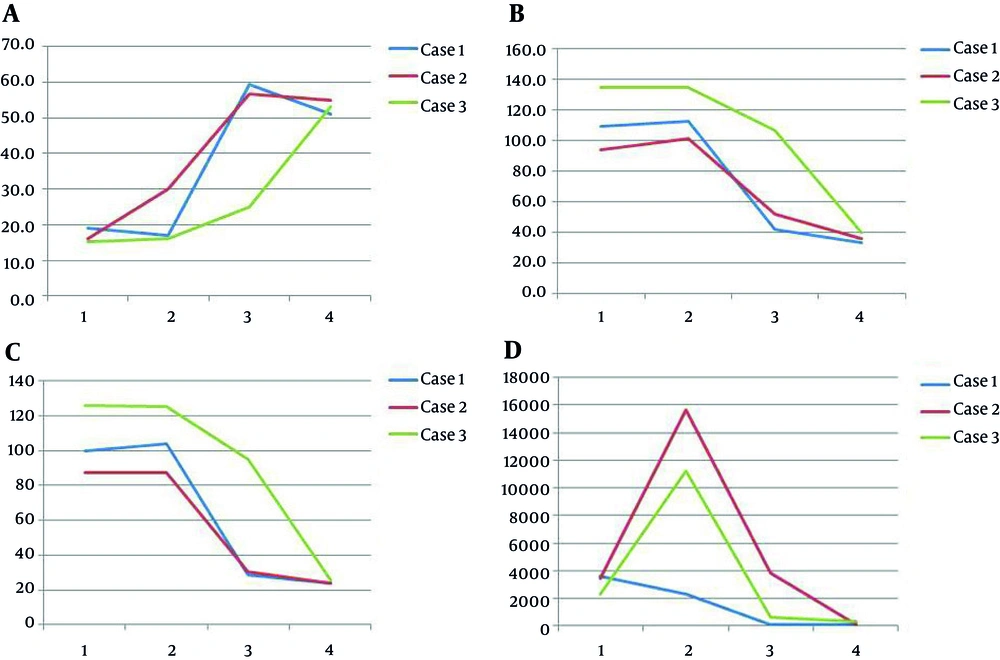
When comparing echocardiographic findings before and after LVVRS, we found that although EF and fractional shortening did not statistically increase, LV end-diastolic dimension (LVEDD), LV end-systolic dimension (LVESD), LVEDD per body surface area (BSA, m2), and LVEDD per BSA significantly decreased after LVVRS (Table 2). The mean follow-up period after LVVRS including failure groups was 84 ± 90.86 months (median of 62 months), and the mean interval from LVVRS to LV failure was 39.29 ± 66.19 months (median of one month, ranging from 1 day to 149 months). Four patients expired within two months after LVVRS, and three patients underwent heart transplantation within two months after LVVRS. Three patients were alive throughout the study period and are doing relatively well, classified as NYHA class II with several medications. The mean follow-up period of three transplant-free survivors after LVVRS was 173.9 ± 26.1 months (ranging from 166 to 217 months). The causes of failure of LVVRS included pump failure (n = 5, 71.4%) and ventricular tachycardia (n = 2, 28.6%). There was no statistically significant difference between the failure and survival groups from the standpoint of age at the diagnosis, preoperative ventricle function, and preoperative pulmonary arterial pressure at cardiac catheterization. LVESD per BSA values (mm/m2) were lower in the failure group than in the survival group at the time of diagnosis of idiopathic DCMP (67.34 ± 17.54 vs. 104.54 ± 19.58, P = 0.048, Table 3). In our study, early mortality was relatively high (40.0 %), but when considering the mean follow-up period (173.9 ± 26.1 months), three patients are alive and are doing relatively well and therefore, we think the midterm results of LVVRS are favorable. In addition, we showed the serial changes in EF, LVEDD/BSA, LVESD/BSA, and the level of NT-proBNP at the diagnosis, pre-operation, post-operation, and at the final follow-up of the survival group (Figure 2).
| Variables | Preoperative Echocardiography | Postoperative Echocardiography | P Value | ||
|---|---|---|---|---|---|
| Mean ± SD | Min-Max | Mean ± SD | Min-Max | ||
| EF by M-mode (%) | 22.49 ± 5.31 | 16.00 - 30.00 | 34.69 ± 18.08 | 12.00 - 59.40 | 0.063 |
| EF by Simpson (%) | 21.48 ± 4.35 | 15.90 - 29.00 | 30.51 ± 18.16 | 14.00 - 60.40 | 0.500 |
| FS (%) | 11.43 ± 2.97 | 7.30 - 14.60 | 18.29 ± 9.04 | 10.00 - 31.70 | 0.063 |
| MR severity | 0.059 | ||||
| N = 7; ≥ moderate | N = 7; ≤ mild | ||||
| N = 2; mild | N = 2; moderate | ||||
| N = 1; unknown | N = 1; unknown | ||||
| LVEDD (mm) | 63.69 ± 12.65 | 42.30 - 80.00 | 55.68 ± 14.43 | 39.10 - 82.00 | 0.025a |
| LVEDD/BSA (mm/m2) | 89.83 ± 30.57 | 45.71 - 134.74 | 64.86 ± 24.32 | 42.12 - 107.05 | 0.017a |
| LVESD (mm) | 57.16 ± 11.56 | 36.50 - 72.00 | 45.68 ± 16.12 | 24.50 - 77.00 | 0.017a |
| LVESD/BSA (mm/m2) | 80.84 ± 28.61 | 41.14 - 125.00 | 53.58 ± 24.44 | 28.81 - 94.77 | 0.017a |
Echocardiographic Findings Before and After Left Ventricular Volume Reduction
| Variables | LVVR Failure (+): N = 7 | LVVR Survival (-): N = 3 | OR | P Value |
|---|---|---|---|---|
| Age at diagnosis (months) | 78.14 ± 40.89 | 28.00 ± 34.78 | - | 0.117 |
| EF at diagnosis (%) | 25.59 ± 10.36 | 16.70 ± 2.04 | - | 0.183 |
| FS at diagnosis (%) | 13.10 ± 5.91 | 7.57 ± 1.25 | - | 0.143 |
| LVEDD/BSA at Dx. (mm/m2) | 76.68 ± 19.66 | 112.98 ± 20.68 | - | 0.095 |
| LVESD/BSA at Dx. (mm/m2) | 67.34 ± 17.54 | 104.54 ± 19.58 | - | 0.048a |
| ≥ moderate MR at Dx, No. (%) | 7 (100) | 3 (100) | - | - |
| EF at pre-OP (%) | 23.23 ± 4.33 | 21.00 ± 7.81 | - | 0.548 |
| FS at pre-OP (%) | 12.42 ± 2.32 | 9.77 ± 3.68 | - | 0.250 |
| LVEDD/BSA at pre-OP (mm/m2) | 76.54 ± 27.30 | 116.40 ± 16.90 | - | 0.095 |
| LVESD/BSA at pre-OP (mm/m2) | 68.53 ± 24.96 | 105.44 ± 18.79 | - | 0.095 |
| ≥ moderate MR at pre-OP, No. (%) | 5 (71.43) | 2 (66.67) | 2.50 | 1.000 |
| LVEDP at pre-OP (mmHg) | 23.50 ± 0.71 | 21.50 ± 9.19 | - | 1.000 |
| RA saturation at pre-OP (%) | 46.70 ± 8.55 | 66.00 ± 0.00 | - | 0.200 |
| PAWP at pre-OP (mmHg) | 30.00 ± 1.41 | 18.50 ± 3.54 | - | 0.333 |
| Age at OP (months) | 104.14 ± 71.14 | 34.67 ± 32.39 | - | 0.117 |
| LV volume reduction with MAP, No. (%) | 5 (71.43) | 3 (100) | 1.40 | 1.000 |
| EF at post-OP (%) | 25.45 ± 11.95 | 47.00 ± 19.19 | - | 0.229 |
| FS at post-OP (%) | 13.88 ± 4.64 | 24.17 ± 11.04 | - | 0.400 |
| LVEDD/BSA at post-OP (mm/m2) | 63.57 ± 20.40 | 67.01 ± 35.02 | - | 1.000 |
| LVESD/BSA at post-OP (mm/m2) | 54.96 ± 18.16 | 51.28 ± 37.67 | - | 0.571 |
| ≥ moderate MR at post-OP, No. (%) | 0 (0.00) | 1 (33.33) | 0.67 | 0.375 |
Differences Between the LVVR Failure and Survival Groups
Serial changes (1; at diagnosis, 2; preoperation, 3; postoperation, 4; at the final follow up) of ejection fraction (A), LVEDD/BSA (B, mm/m2 ), LVESD/BSA (C, mm/m2), and the level of NT-proBNP (D, pg/mL) in the alive groups. LVEDD, left ventricular end-diastolic dimension; BSA, body surface area; LVESD, left ventricular end-systolic dimension; NT-proBNP, N-terminal fragment of the prohormone brain-type natriuretic peptide.
5. Discussion
Although high mortality after LVVRS was noted in children with idiopathic DCMP, some patients had favorable mid-term outcomes according to the present study. We report that LVVRS might be considered as a bridge therapy to heart transplantation in children and adolescents.
LVVRS as an organ-preserving operation was originally proposed to reduce the diameter of the dilated left ventricle by excising a sizable amount of the ventricular free wall and was popularly performed in the mid-1990s. However, conventional PLV was largely abandoned by the year 2000 in western countries because of unexpected high mortality and poor long-term results (11). Although the results of our experience still suggest a relatively high rate of early cardiac death (n = 4; 40.0%), when considering the mean follow-up period (173.9 ± 26.1 months), three patients are alive and are doing relatively well and therefore, we think the midterm results of LVVRS are favorable. Although the total number of patients is very small, we would like to emphasize that LVVRS has favorable mid-term survival results for idiopathic DCMP in children and adolescents. LVVRS might be a valuable bridge therapy when patients do not meet the criteria for transplantation, have medical contraindications, or are limited due to donor supply. Our technique of carrying out LVVRS through a small apical incision reduces the LV volume without excision of the myocardium. There might be several advantages for this less invasive trans-apical approach compared to PLV. First, minor damage to myocardial fibers and minimal injury to the coronary artery may reduce the incidence of myocardial dysfunction, myocardial fibrosis, and ventricular arrhythmia in late follow-up periods. Second, the risk of postoperative bleeding is minimal. Third, the original state can be restored if the patient cannot tolerate trans-apical LVVRS.
The literature review and our results suggest that many factors might explain the significant differences observed in early and late clinical outcomes following LVVRS.
First, patient selection is an important factor. Preoperative hemodynamic instability might be associated with early mortality after LVVRS. Late outcomes also are influenced by patient selection. Vural and Tasdemi classified NYHA functional class IV, congestive hepatomegaly, LV end-diastolic pressure > 25 mmHg, left atrial diameter > 55 mm, and pulmonary artery systolic pressure > 40 mmHg as poor prognostic predictors of late mortality (12). The recurrence of congestive heart failure (low cardiac output) was one of the most common causes of late death in the literature and in our study.
Second, the type of surgery might influence long-term results. In the Batista procedure, asymmetrical resection of the affected ventricular free wall can result in different lengths of the two resected margins. Therefore, some areas are stretched more than others are while suturing and this can lead to unpredictable ventricular shape, which might also affect coronary arterial perfusion (13-15). Dor et al. emphasized the reconstruction of a more elliptical LV cavity and treatment of all components of dilation (anterior, apical, and septal) while reducing LV size (16).
Third, the accurate correction of left ventricular compliance is important. Because the site, shape, and size of the resected segments depend on the surgeon’s judgment during the operation and the unwillingness to exclude akinetic segments that appear normal on the surface, there can be over-correction or under-correction of LV compliance.
Forth, intractable arrhythmias, especially ventricular tachycardia, influence the long-term survival of patients with LVVRS. Approximately, 10% of late deaths were reported as a result of malignant arrhythmias in stable patients with NYHA functional class I or II symptoms after LVVRS (3). Similarly, one of the findings of our observational analysis was that two patients who expired early (n = 4) passed away due to ventricular tachycardia and fibrillation.
Fifth, according to previous studies, the recurrence of late cardiac failure appears to be related to the development of progressive mitral insufficiency at follow-up (17, 18). Therefore, LVVRS is usually combined with mitral valve and tricuspid valve reconstruction with an edge-to-edge procedure (17, 18). We also performed mitral valve (n = 8) and tricuspid valve (n = 4) reconstruction including valve replacement in our patients including all three survivors.
There were several limitations to this study. This study had a retrospective design, it was performed at a single hospital, and it included a small number of patients. Therefore, the results of this study might not allow the generalization to the overall pediatric population. This study is somehow outdated that is another limitation of this study because of the rarity of idiopathic dilated cardiomyopathy in children. Actually, heart transplantation is the gold standard therapy in the terminal heart failure state in children in spite of several problems such as the rarity of donors and high costs. Although the left volume reduction surgery has several problems, we emphasize based on the current study that this technique might be a good bridge surgery to the heart transplantation in children.
5.1. Conclusion
Although high early mortality after LVVRS was noted in children with idiopathic DCMP, some patients had favorable mid-term outcomes. We suggest that LVVRS might be considered as a bridge therapy to heart transplantation in children and adolescents.