1. Background
Respiratory distress is one of the most common neonatal diseases and approximately 1% of all newborns experience some degrees of respiratory distress. Among all hospitalized infants due to respiratory distress, especially those admitted to neonatal intensive care unit, one third are finally detected with transient tachypnea of the newborn (TTN) (1, 2). Natural respiratory distress caused by TTN is usually resolved during 48 - 72 hours after birth, but it may last up to 5 days. Although little is known about the pathology of TTN, this is a respiratory disorder caused by insufficient or delayed clearance of neonate alveolar fluid (3, 4). Lung fluid absorption is beginning by beta-adrenergic agonists, such as endogenous steroids and catecholamines, which increase during childbirth. Neonates who do not have TTN in comparison with those with this disorder, face with lower levels of circulating catecholamines and also experience a delay or reduction in absorption of fluid by their alveoli (5-7). Both human and animal experimental condition models have shown that stimulation of lung tissue with extrinsic β-adrenergic agonists causes an increase in lung fluid absorption. In addition, it was recently shown that intravenous administration of Albuterol (Salbutamol), a β2 - adrenergic agonist, stimulated the lung fluid absorption (7-9). TTN is often benign and self-limited, but in some cases can lead to respiratory failure. So far, no effective drug therapy was introduced for the treatment of TTN and its standard treatment is the use of supplemental oxygen and respiratory support but severe resistant cases also need intubation and mechanical ventilation. Respiratory support with intubation has a lot of side effects such as changes in the blood pressure, intraventricular hemorrhage, and also respiratory support with mechanical ventilation is accompanied with numerous complications (10). Accordingly, we hypothesized that nebulized Salbutamol (albuterol), may enhance lung fluid absorption in patients with TTN and might be effective in reduction of respiratory symptoms of these neonates.
2. Objectives
The main objective of this study was to evaluate the effectiveness of nebulized albuterol in reducing respiratory distress as well as the neediness to respiratory continuous positive airway pressure (CPAP) support in infants suffering from TTN. Our secondary objective was to assess the safety and possible side effects of nebulized albuterol in the treatment of TTN.
3. Methods
3.1. Subjects
All neonates admitted with TTN and respiratory distress score > 4- < 10 to neonatal intensive care unit (NICU) of Taleghani hospital in Tabriz, Iran from August to December 2015 were included in the study. Infants with transient tachypnea of the newborn were characterized based on the following clinical criteria:
1) At least 35 weeks gestational age, 2) presence of respiratory distress less than 6 hours after birth (respiratory rate more than 60/min, cyanosis, grunting, nasal flaring, or retraction), and 3) typical chest radiography findings (fluid in minor fissures, hyperinflation, prominent vascular/perihilar markers) (11).
Patients who had any of the following items were excluded: 1) meconium aspiration. 2) Other causes of tachypnea (ie, respiratory distress syndrome, persistent pulmonary hypertension, pneumonia, sepsis, neonates under the age of 35 weeks, polycythemia, or hypoglycemia), 3) congenital heart diseases. and 4) tachycardia (heart rate more than 180/min) or arrhythmia, 5) Infants with respiratory distress scores less than 5 and more than 10.
Criteria for ruling out other common causes of respiratory distress included:
1. Respiratory distress syndrome: when no patterns of reticulogranular or white lung with decreased aeration were seen in the chest’s radiography or surfactant has not been used.
2. Meconium aspiration syndrome: when no history of meconium disposal or abnormal chest x-ray findings (irregular density pattern in the lung) and skin, amniotic fluid meconium staining were not found.
3. Persistent pulmonary hypertension of the newborn: when the difference between preductal /postductal oxygen saturation was < 5%.
4. Sepsis: When no prenatal infection risk factors and, white blood cells (WBC) less than 5000 or more than 15,000/mm3, immature to total neutrophils ratio (TI) > 0.25, positive C-reactive protein and focal infiltrate on chest radiography did not exist.
In this study, tachypnea and tachycardia were defined as respiratory rate > 60 breaths/min, and heart rate > 180 beats/min respectively.
3.2. Study Design
Neonates that had been hospitalized immediately after birth for transient tachypnea of the newborn were divided into two groups based on their ACORN respiratory distress scores:
| Score | 0 | 1 | 2 |
|---|---|---|---|
| Respiratory rate | 40 to 60/minute | 60 to 80/minute | > 80/minute |
| Oxygen requirement | None | ≤ 50% | > 50% |
| Retraction | None | Mild to moderate | Severe |
| Grunting | None | With stimulation | Continuous at rest |
| Breath sounds on auscultation | Easily heard throughout | Decreased | Barely heard |
| Gestational age | > 34 weeks | 30 to 34 weeks | < 30 weeks |
aAdapted from Downes JJ, Vidyasagar D, Boggs TR Jr, Morrow GM 3rd. Respiratory distress syndrome of newborn infants. I. New clinical scoring system (RDS score) with acid-base and blood-gas correlations. Clin Pediatr 1970; 9 (6): 325 - 31.
Infants with mild respiratory distress (respiratory scores less than or equal to 4) received oxygen therapy via oxyhood, and neonates with severe respiratory distress (respiratory scores ≥ 10) with evidence of respiratory failure were intubated and excluded.
Infants with respiratory scores of ≥ 5 and < 10 were included in study and firstly received continuous positive airway pressure support (CPAP), with PEEP: 5, FIO2: 0.3 and then based on their partial pressure oxygen (PO2) or oxygen saturations (SO2), the neediness to oxygen demands and positive pressure was regulated. In this study oxygen saturation was set on 92% - 95%.
All enrolled neonates were randomly (computer-generated) allocated into 2 groups of treatment and placebo. Treatment group received 0.15 mg/kg albuterol (Salbutamol sulfate 5 mg/mL, Glaxo Smith Kline, UK) in 2 mL of normal saline, and placebo group received 2 mL of normal saline every 6 hours for 24 hours. Each dose of drug or placebo was nebulized within 10 minutes by jet nebulizer (Omron Micro Air NE-U22E- Japan) through the input arm CPAP set. The oxygen concentrations, based on the needs of infants, were adjusted to maintain the oxygen saturation above 92% in all neonates. Both drug and placebo were prepared and presented similarly in shape and color coded by a single person who was not involved in infants’ care. It was agreed that in case of tachycardia or arrhythmia, at any time, the patient treatment must be terminated immediately. All demographic data of neonates were recorded in the prepared forms, and the following clinical parameters were evaluated both before starting the study, and after application of placebo/albuterol, every 6 hours for 24 hours: Respiratory distress score (using ACORN), arterial blood gas (ABG), heart rate, heart rhythm, duration of CPAP support and the necessity of using ventilator support. To evaluate the safety or possible side effects of treatment, all neonates were monitored for tachycardia and arrhythmia.
3.3. Statistics
Sample size was calculated with 95% confidence interval, a power of 80% and standard deviation of 0.20, and a total sample size of 50 neonates was estimated, namely at least 25 neonates in each arm. However, during the study period and based on convenience sampling method 60 near term/term neonates aged 0 to 6 hours with signs of TTN were identified and whole this figure was selected and assigned to the defined groups by staffs who were not involved in the infant’s care, randomly.
SPSS 20.0 for windows (SPSS Inc., Chicago, IL, USA) was used for analysis. Values were shown as averages ± standard deviation via tables and diagrams appropriately. To compare the frequency and proportion of categorical variables, Chi-squared test (Fisher’s exact test) was used. Student’s t-test was also used to analyze differences between the averages of 2 independent groups. For all statistical tests, the significant level was considered as P ≤ 0.05.
3.4. Ethics Considerations
Objectives and methods were explained to the parents prior to entering the study and informed consent was obtained from the parents of all neonates. The study design was approved by the ethics committee of Tabriz University of Medical Sciences, with IRB number TBZMED.REC.1394.701. The project was registered via Iranian registry of clinical trials and a registration ID was allocated as IRCT201305188680N3.
4. Results
4.1. Characteristics of Study Patients
A total of 60 infants were included in this study: 30 patients were randomly allocated into the treatment group (albuterol nebulization); and 30, into the placebo group (normal saline nebulization). All enrolled neonates completed all steps of the study (Figure 1).
The average gestational age of all patients was 36.9 ± 1.69 weeks, and the birth weight was 2703 ± 589 gr. Thirty one (51.7%) of the babies were male and thirty two (53.3%) babies were delivered by cesarean section. The average initial respiratory distress score was 5.6 ± 1.64, and their Apgar score was 8.4 ± 0.49 at either 1 or 5 minutes. There were no significant differences between the treatment and placebo groups considering any of the following variables: gestational age (37 ± 1.74 weeks vs 36.8 ± 1.66 weeks), birth weight (2676.3 ± 611.1 gr vs 2731.0 ± 576.4 gr), delivery method (vaginal delivery vs cesarean section), 1 minute Apgar score, 5 minutes Apgar score, initial respiratory distress score, initial heart rates, blood gas test results, and medical history of mother (Tables 2 and 3).
| Variable | Study Group | Control Group | Total | P | |||
|---|---|---|---|---|---|---|---|
| Number | % | Number | % | Number | % | ||
| Gender | 0.15 | ||||||
| Male | 13 | 42 | 18 | 58 | 31 | 51.7 | |
| Female | 17 | 58.6 | 12 | 41.4 | 29 | 48.3 | |
| Delivery | 0.09 | ||||||
| Vaginal | 17 | 60.7 | 11 | 39.3 | 28 | 46.7 | |
| S/C | 13 | 40.6 | 19 | 59.4 | 32 | 53.3 | |
| Maternal diabetes | 0.5 | ||||||
| Yes | 3 | 60 | 2 | 40 | 5 | 8.3 | |
| No | 27 | 49.1 | 28 | 50.9 | 55 | 91.7 | |
| Maternal asthma | 0.5 | ||||||
| Yes | 1 | 100 | 0 | 0 | 1 | 1.7 | |
| No | 29 | 49.1 | 30 | 50.9 | 59 | 98.3 | |
| PROM | 0.67 | ||||||
| Yes | 3 | 50 | 3 | 50 | 6 | 10 | |
| No | 27 | 50 | 27 | 50 | 54 | 90 | |
| Mechanical ventilation need | 0.75 | ||||||
| Yes | 1 | 50 | 1 | 50 | 2 | 3.3 | |
| No | 29 | 50 | 29 | 50 | 58 | 96.7 | |
Abbreviations: PROM, Preterm rupture of membrane; S/C, Cesarean section.
| Variable | Study Group | Control Group | Total | P | |||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | ||
| Weight (gr) | 2676.3 | 611.1 | 2731.0 | 576.4 | 2703.67 | 589.57 | 0.72 |
| GA (weeks) | 37.0 | 1.74 | 36.87 | 1.66 | 36.9 | 1.69 | 0.76 |
| Apgar score (1 minute) | 8.2 | 0.4 | 8.57 | 0.5 | 8.4 | 0.49 | 0.008 |
| Apgar score (5 minutes) | 8.2 | 0.4 | 8.57 | 0.5 | 8.4 | 0.49 | 0.008 |
| CPAP duration (day) | 1.6 | 0.77 | 3.3 | 0.98 | 2.4 | 1.2 | 0.0001 |
| PH, at start study(mm/hg) | 7.34 | 0.095 | 7.35 | 0.1 | 7.3 | 0.098 | 0.82 |
| PH, at end study (mm/hg) | 7.38 | 0.071 | 7.4 | 0.12 | 7.39 | 0.1 | 0.38 |
| PCO2, 6 hours after delivery (mm/hg) | 40.5 | 9.8 | 40.9 | 9.5 | 40.7 | 9.6 | 0.87 |
| PCO2, 12 hours after delivery (mm/hg) | 43.97 | 10.3 | 40.2 | 10.6 | 42.1 | 10.6 | 0.17 |
| PCO2, 18 hours after delivery (mm/hg) | 43.97 | 9.6 | 39.6 | 13.2 | 41.8 | 12.2 | 0.15 |
| PCO2, 24 hours after delivery (mm/hg) | 43.03 | 7.9 | 40.9 | 14 | 41.97 | 11.4 | 0.47 |
4.2. Effects of Nebulized Ventolin on Study Variables
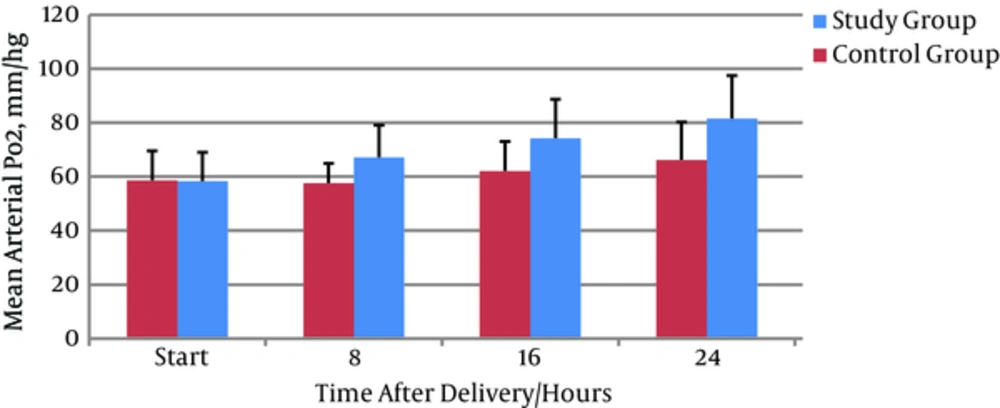
The duration of CPAP application was significantly decreased in the treatment group compared to the placebo group (1.6 ± 0.77 vs 3.3 ± 0.98 respectively) (P = 0.0001) (Table 3). There were no significant differences between the treatment and placebo groups in initial PO2 (58.3 ± 10.72 vs 58.57 ± 10.96 mmHg respectively) but arterial oxygen (PO2) was significantly more increased in the treatment group (84.8 ± 10.3 mmHg) than in the placebo group (70.17 ± 17.14 mmHg (P < 0.001) (Figure 2). Initial and final PCO2 did not differ between the two groups, but it was increased in the treatment group 6 hours after initiation of albuterol nebulization (P = 0.97) (Table 3).
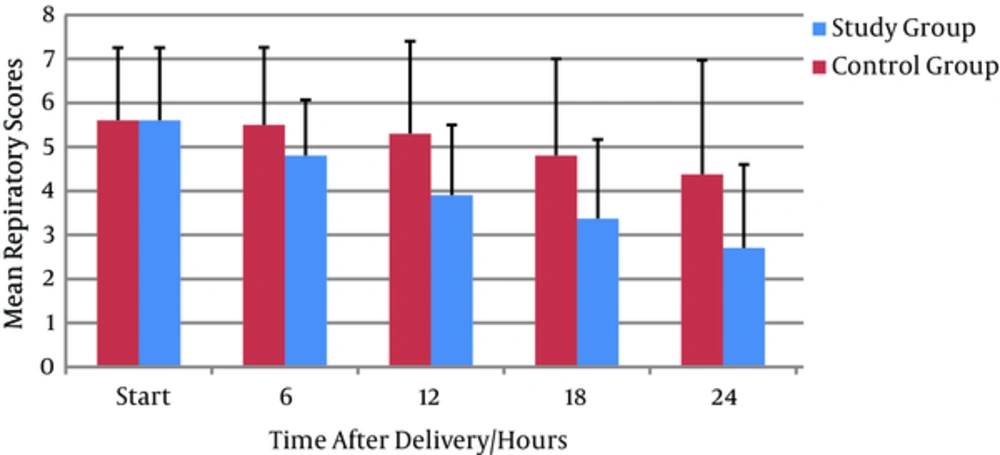
Comparing respiratory distress score showed no differences in baseline in both groups; however, in the treatment group, it was decreased significantly over the course of 6 hours onward to 36 hours (1.7 ± 2.0 vs 3.9 ± 2.89; P = 0.001) suggesting that the albuterol caused a significant improvement in TTN symptoms and a considerable reduction in respiratory scores at its primary hour of application (Table 3, Figure 3).
4.3. Tolerability of Nebulized Ventolin
The most important side effects of albuterol were tachycardia (heart rate > 180 beats/min) and arrhythmias. No such effects were observed for either the treatment or placebo groups in this study (Figure 4).
5. Discussion
Transient tachypnea is a common physiologic disorder caused by pulmonary edema secondary to insufficient or delayed absorption of alveolar fluid. This disorder, with a prevalence rate of 5.7 per 1,000 newborns, can be seen more frequently in males, term or near term neonates born via caesarean section, and babies of mothers with diabetes or asthma. Clinical manifestations of TTN are tachypnea, grunting, nasal flaring, and intercostal muscles retraction soon after birth. These symptoms usually resolve spontaneously within 48 - 72 hours after birth, but may last up to 5 days. During pregnancy fetal alveolar fluid secreted continuously through a chloride secretion mechanism of epithelium and the secretion rate is reduced few days before delivery. At the onset of labor and birth the balance of fluid in the alveoli changes from chloride secretion to sodium chloride absorption in the alveoli, causing alveolar fluid absorption. Sodium is initially transferred through a concentration gradient by sodium-potassium pumping passively into the cells of alveolar lumen and in the next step is actively transmitted into the interstitial space by amiloride sensitive epithelial sodium channel. After that, sodium and fluid are cleaned through the lymph system and blood vessels (1-4). Hormonal changes associated with vaginal delivery, especially increased endogenous catecholamines and steroids, seem to increase ENAC activity, and reduce the incidence of TTN (9). In addition, it was recently shown that administration of albuterol (Salbutamol), a β2- adrenergic agonist, stimulates the lung fluid absorption (11).
Based on these reports, we hypothesized that albuterol stimulates lung fluid absorption in infants with TTN may be useful in reducing the neonatal respiratory distress. Nebulized formulations with minimal systemic effects effectively enter into the lung. In addition, safety and efficacy of this approach is well-proven and this method is usually used in the treatment of bronchopulmonary dysplasia in premature infants. Accordingly, the potential risk associated with systemic therapy of β2- adrenergic agonist cannot be of concern.
Our study showed that the final respiratory distress score was significantly decreased in the treatment group than in the placebo group. Also considering no significant differences between the treatment and placebo groups in initial PO2 but arterial oxygen (PO2) was significantly increased in the treatment group than in the placebo group at the end of study. Since decreasing respiratory distress score and increasing the arterial oxygen pressure causes a reduction in the oxygen concentration, either the duration or concentration of the applied oxygen is also declined because the oxygen demand was one of respiratory score parameters.
By reduction of respiratory scores and severity of respiratory distress, the necessity of using positive pressure of airways was also reduced and therefore duration of CPAP application was declined too (Table 3).
Initial and final PCO2 did not differ between the 2 groups, but it was increased in the treatment group 6 hours after initiation of albuterol nebulization, probably due to a decrease in the respiratory rates.
In a similar research, Kim MJ and colleagues investigated the effects of inhaled Salbutamol therapy in improving clinical symptoms in neonates with TTN via a prospective study of 40 infants admitted to a neonatal intensive care unit. Their study showed that the duration of tachypnea was lower in patients receiving inhaled Salbutamol, but statistically insignificant. They also reported that the duration of treatment with supplemental oxygen and empirical antibiotic treatment in patients treated with Salbutamol was significantly shorter and no side effects were observed in both groups (11). Similar to our results their findings showed no side effects in using inhaled β2- adrenergic agonist among TTN babies.
Another study by Armangil and colleagues examined the effects and possible side effects of inhaled Salbutamol in treatment of TTN among 54 infants with gestational age of 34 - 39 weeks. They reported a significant reduction in the need for respiratory support level, oxygen requirement and respiratory rate in Salbutamol group 30 minutes after inhalation. They also indicated that the levels of PCO2, PO2 in the Salbutamol group were better than the control group (12). Their findings were in accordance with the results of our study.
Our study showed that application of nebulized albuterol caused a considerable improvement in the respiratory distress of neonates and PO2 at its primary hour of using via reduction in the need for respiratory support level and duration of oxygen supplementation. This issue may be due to nebulization intervals (every 6 hours) which with increasing Ventolin doses or decreased interval (every 4 hours) may render better effects.
5.1. Conclusions
There was limited study to show the effects of albuterol therapy on respiratory distress score, duration of positive pressure respiratory support and albuterol complications.
Our study findings suggest that albuterol nebulization therapy significantly decreases respiratory distress score, increases PO2, and decreases the oxygen demand and CPAP requirements in neonates with TTN (score ≥ 5 ≤ 10), without considerable side effects.
Further researches are needed to examine the effects of nebulized albuterol on TTN in a larger number of infants and whole spectrum of TTN (mild to severe) and measuring the serum level of albuterol is also necessary to fully characterizing the effects of this treatment on TTN.