1. Background
Parasitic infections have been reported in about 25% of the world’s population. These infections are more prevalent in developing countries, especially in rural areas of tropical and subtropical regions (1, 2). In Egypt, parasitic diseases represent a serious public health problem in both immunocompetent and immunocompromised patients with clinical and economic impacts. The prevalence of parasitic infections among children is high, with levels reaching up to 50%. This high prevalence is mainly due to overcrowding and poor hygiene patterns that significantly contributed to the spread of parasitic infections (1-3).
Allergic rhinitis (AR) is the most common airway allergic disease. Nowadays, AR is a significant health problem affecting up to forty percent of children worldwide, which have a significant influence on children’s quality of life, costs involved, and load on national health care services. AR is an allergic inflammation of the nasal mucous membranes. It is a type I hypersensitivity reaction mediated by allergen-specific IgE antibodies, presented by repetitive paroxysmal sneezing, watery rhinorrhea, and nasal blockage. AR often shares the common risk factors for bronchial asthma, especially atopy (4). Genetic and environmental risk factors are involved in the pathogenesis of AR; however, the exact etiology remains to be identified (5, 6). Epidemiological studies conducted in many countries have reported that helminthic infections are, associated with a reduced or increased prevalence of atopy and allergic diseases (7, 8). Total IgE, IL-5 and leukotriene E4 may be involved in the pathogenesis of atopic and allergic disorders, and patients with allergy may have enhanced levels of both compared with normal individuals (8, 9). Although there have been many recent studies, the association between helminthic infections and childhood atopy remains controversial (5).
2. Objectives
Because of the conflicting results in the literature on parasitic infections in pediatric allergic disorders, especially AR and the number of the studies are very few; the present study aimed to evaluate parasitic infections in children with AR referring to a tertiary care center in Upper Egypt.
3. Methods
3.1. Study Design
This was a case-control study undertaken in Assiut Children University Hospital, Assiut, Upper Egypt.
3.2. Participants
This study included 139 children with AR attending Assiut University Children’s Hospitals, Egypt from January 2014 to May 2016. The diagnosis of AR was established by a senior ENT consultant before recruitment of patients into the study. Diagnosis of AR was confirmed according to Allergic Rhinitis and Its Impact on Asthma (ARIA) guidelines (10). Severity grading and classification of AR patients were performed based on ARIA criteria as follows: Intermittent AR (mild or moderate-severe) or persistent AR (mild or moderate-severe) (10). All manifestations of AR including sneezing, rhinorrhea, nasal obstruction, and itching, and facial features (allergic salute, shiners, nasal crease, mouth breathing, conjunctivitis, and infraorbital fold) were assessed in all patients. Seventy age- and sex-matched non-atopic, non-asthmatic healthy children were recruited as healthy controls. The controls were enrolled after exclusion of chronic systemic diseases or family history of atopy. Treatment with systemic antihistamines and corticosteroids was stopped at least two weeks before the study. Children were excluded from the study if they had any of the following: 1) manifestations of allergic symptom exacerbation, 2) associated other diseases, e.g., sinusitis, asthma, acute respiratory tract infections, and nasal septum deformities, and 3) recent history of anti-parasitic medications or immunotherapy six months before the study.
3.3. Laboratory Investigations
All patients and controls underwent laboratory tests, which included stool examination, total IgE, absolute eosinophilic count (AEC), serology for IgE antibodies to Ascaris lumbricoides, ELISA for IgG antibodies to Toxocara canis, serum IL-5 levels, and leukotriene E4 in urine.
3.3.1. Stool Examination
Stool samples from all participants were collected in sterile clean stool plastic disposable cups with lids labeled with the patient’s serial number, name, age, sex, group of AR and date of collection. The parasitological examinations were immediately processed within half an hour. A direct wet smear was performed using iodine and lactophenol cotton blue. Afterward, fomol-ether sedimentation was done to the stool samples and examined by direct wet smear (as previous) and Kinyoun acid-fast staining procedure.
3.3.2. Urinary Leukotriene E4
LTE4 levels were measured using the commercially available enzyme immunoassay (Cayman Chemical; Ann Arbor, MI, USA).
3.3.3. Blood Samples
Blood samples were collected from the subjects by venepuncture. Cellular assay (AEC) was performed (Eosinophilia corresponded to levels above 400/mm3), and the serum samples collected were stored at -70ºC until the serological analysis.
3.3.4. Levels of Total IgE
The levels of the total IgE were measured by ELISA where levels above 200 IU/mL were considered high. All samples were measured in duplicate.
3.3.5. Human IL-5 Level Assay
IL-5 levels were measured using the commercially available human enzyme-linked immunosorbent assay kit (Biosource International, Inc., Camarillo, California, USA), according to the manufacturer’s instructions. The lowest level of detection of IL-5 was 2 pg/mL. The intra-assay coefficient of variation was 7.4%, and the inter-assay coefficient of variation was 10%.
3.3.6. Serological Detection of Ascaris lumbricoides Infection
Specific IgE levels against Ascaris were measured by the CAP-FEIA fluoro enzyme immunoassay method (Phadia AB, Uppsala, Sweden).
3.3.7. Serological Detection of Toxocara canis Infection
Excretory/secretory antigens were prepared from laboratory cultivated second stage larvae of T. canis. The antigen was stored at -70ºC until used as a crude antigen. IgG against T. canis was detected by ELISA technique. ELISA plates (Flow Lab. Cat. No., 76-321-05) were coated by the prepared antigen. Anti-human IgG peroxidase (Sigma-A 602g) conjugate and orthophenylene diamine substrate buffer citrate (OPD Sigma Cat. No. P-4512) were used.
3.4. Statistical Analysis
Data were analyzed using SPSS statistics version 22 (IBM Corporation, NY, USA). Values were expressed as means and standard deviation (SD). Qualitative variables were presented as number (n) and percentage (%). Chi-square test was used to compare qualitative variables between groups. Unpaired t-test and Mann-Whitney “U” tests were used to compare quantitative variables. Anti-Ascaris IgE was classified into quartiles based on the distribution of the study participants.
4. Results
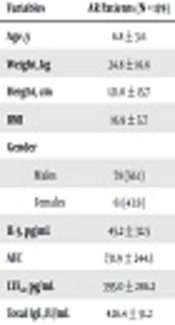
Table 1 shows the demographic and laboratory data for patients and controls. The mean age of the patient group was 6.8 ± 3.6 years, and their age range was 6 - 14 years, with insignificant differences with the control group. AR patients showed significantly higher values of AEC, IL-5 and total IgE than controls. Furthermore, AR children showed significantly higher urinary LTE4 levels than controls (Table 1). In the patient group, 86.3% of the AR children resided in urban districts, while the remaining (13.7%) lived in rural regions. In the control group, 85.7% of children lived in urban areas, while 14.3% lived in rural regions.
| Variables | AR Patients (N = 139) | Controls (N = 70) | P Value |
|---|---|---|---|
| Age, y | 6.8 ± 3.6 | 7.1 ± 2.9 | 0.85 |
| Weight, kg | 24.8 ± 16.6 | 26.1 ± 15.4 | 0.35 |
| Height, cm | 121.0 ± 15.7 | 122.0 ± 14.2 | 0.28 |
| BMI | 16.9 ± 5.7 | 17.2 ± 4.8 | 0.30 |
| Gender | 0.28 | ||
| Males | 78 (56.1) | 40 (57.1) | |
| Females | 61 (43.9) | 30 (42.9) | |
| IL-5, pg/mL | 45.2 ± 32.5 | 6.7 ± 3.9 | 0.0001 |
| AEC | 731.9 ± 244.3 | 121.9 ± 51.6 | 0.0001 |
| LTE4, pg/mL | 395.0 ± 286.2 | 35.2 ± 5.0 | 0.001 |
| Total IgE, IU/mL | 426.4 ± 51.2 | 32.9 ± 14.2 | 0.0001 |
Abbreviations: AEC, absolute eosinophilic count; IL-5, interleukin-5; LTE4, leukotriene E4.
a Values are expressed as mean ± SD or No. (%).
b No significant (P > 0.05).
As regards the severity of AR, patients were classified into two groups; mild and moderate/severe AR. Group I included 78 patients with mild AR (45 males and 33 females) and group II included 61 patients with moderate/severe AR (33 males and 28 females).
As regards the frequencies of parasitic infections among the examined patients and controls; stool examination for Ascaris ova and larvae and anti-Ascaris IgE were positive in 26 (18.7%) patients and seven controls (10%). IgG anti-Toxocara canis was detected in 25 (18 %) patients and five controls (7.1%), whereas Giardia infection was detected in stools of 28 (20.1%) patients and four controls (5.7%). Among 26 patients infected with Ascaris 24 patients had moderate/severe AR, and only two patients had mild AR. Among AR patients infected with Toxocara 21 patients had moderate/severe AR and only four patients had mild AR. Of 28 AR patients infected with Giardia 20 patients had moderate/severe AR, and eight patients had mild AR. AEC, serum IL-5 and urinary LTE4 levels were significantly higher in patients with moderate/severe AR when compared to children with mild AR (Table 2). Patients with positive parasitic infections showed significantly higher values for AEC, serum IL-5, and urinary LTE4 than those with negative infection. We found that AR patients with Giardia infection, anti-Ascaris IgE, and anti-Toxocara IgG were significantly associated with AEC, total IgE, IL5 and Leukotriene E4. No association was found with age, sex, and residence, but a definite association with disease severity.
| Group I Mild AR (N:78) | Group II Moderate/Severe AR (N:61) | Group III Controls (N:70) | P1-Valuei Versus III | P2-Value II Versus III | P3-Value Versus II | |
|---|---|---|---|---|---|---|
| AEC | 332.6 ± 100.6 | 953.5 ± 122.0 | 121.9 ± 51.6 | 0.001 | < 0.0001 | 0.0001 |
| IL-5, pg/mL | 13.3 ± 3.6 | 74.3 ± 30.3 | 6.7 ± 3.9 | 0.001 | < 0.0001 | 0.0001 |
| LTE4, pg/mL | 110.1 ± 49.4 | 656.3 ± 259.7 | 35.2 ± 5.0 | 0.001 | < 0.0001 | 0.0001 |
Abbreviations: AEC, absolute eosinophilic count; IL-5, interleukin-5; LTE4, leukotriene E4.
a Values are expressed as mean ± SD.
5. Discussion
The incidence of allergic diseases in children, e.g., allergic rhinitis, asthma, and atopic eczema has been continuously rising, especially over the last two decades (11). Given the influence on children’s quality of life, financial impact, and considerable load on the national health care services, there have been numerous clinical studies to recognize the predisposing factors and treatment modalities of allergic diseases. The link between helminthic infections and childhood atopy remains controversial. In this study, we investigated the prevalence and relation of parasitic infections with Ascaris lumbricoides, Toxocara canis, Giardia lamblia and the severity of AR in children compared to healthy children. As regards the association of parasitic infections (ascariasis, toxocariasis, and giardiasis) and AR, anti-Ascaris IgE and IgG anti-Toxocara canis were detected in 18.7% and 18% of patients, respectively, whereas Giardia infection was detected in 20.1% of our cohort. All the three types of parasitic infections were significantly higher in AR patients when compared to control group (P = 0.01 for each). Moreover, parasitic infections with Ascaris, Toxocara, and Giardia were more common among moderate/severe AR children than mild AR. This was supported by the detected significantly higher AEC, urinary LTE4 and IL-5 in Ascaris (Table 3), Toxocara and Giardia positive AR than negative cases.
| I Patients with Positive Ascaris Infection (N: 26) | II Patients with Negative Ascaris Infection (N: 113) | III Controls (N: 70) | P Values | |||
|---|---|---|---|---|---|---|
| I Versus III | II Versus III | I Versus II | ||||
| AEC | 888.0 ± 249.7 | 696.3 ± 230.8 | 121.9 ± 51.6 | 0.0001 | 0.0001 | 0.010 |
| IL-5, pg/mL | 62.7 ± 37.4 | 41.2 ± 30.3 | 6.7 ± 3.9 | 0.0001 | 0.0001 | 0.031 |
| LTE4, pg/mL | 665.8 ± 308.5 | 340.9 ± 253.5 | 35.2 ± 5.0 | 0.0001 | 0.001 | 0.009 |
Abbreviations: AEC, absolute eosinophilic count; IL-5, interleukin-5; LTE4, leukotriene E4.
a Values are expressed as mean ± SD.
In line with our results; Dold et al. (12), studied Ascaris-specific IgE and allergic sensitization in a large cohort of atopic children in East Germany (2300 children) in two surveys. They reported that seropositive Ascaris-IgE had tenfold higher levels of total IgE than negative patients. Furthermore, these patients had higher prevalence rates of seropositive allergen-specific IgE. Also, they had a higher prevalence of allergic rhinitis (P < 0.001) and asthma (P < 0.05). After adjustment for age, sex and other variables, a positive result of Ascaris IgE was a stronger risk factor for allergic sensitization to inhalant allergens. The study reported that low doses of parasitic antigens are accompanied with an increase of IgE production, and parasitic infestations were not causative factors for the low prevalence of allergies in East Germany (12). The observations about Ascariasis and allergy were reported by many epidemiological studies, using numerous approaches. These reports have shown that Ascariasis is a risk factor for atopic diseases (13-16). Not all studies, however, have shown an association between Ascariasis and allergy, some reports have indicated inverse associations between allergen skin test reactivity and infections with Ascariasis (17, 18).
As regards Toxocara infection, the pieces of evidence from the previous epidemiological studies were also conflicting. In agreement with our research, most of the studies (19-21) suggested that Toxocara infection contributed to the development of atopy and allergic disorders. Chan et al. (19) reported that Toxocara infection might increase the predisposition to atopy and allergic diseases, especially in children. Furthermore, toxocariasis was associated with higher levels of total serum IgE, allergen-specific IgE, eosinophil counts, atopic asthma, increased skin sensitivity to aeroallergens, and reduced lung functions in the Toxocara seropositive patients than in the seronegative group (19-21). On the other hand, not all studies supported the previous findings. Few studies were unable to show any positive association between Toxocara seropositivity and allergen skin test reactivity and atopic diseases (22, 23).
Few studies investigated the association between giardiasis infection and allergic diseases. In line with our study, Di Prisco et al. (24) reported that 70% of the patients infected with G. lamblia presented with allergies, such as allergic rhinitis, asthma, atopic dermatitis, angioedema, acute urticaria, and chronic urticaria, compared to only 43% of non-parasitized patients. The authors attributed the increase of these allergic diseases to changes in intestinal mucosal cells of the infected host, which may favor the absorption of non-adequately metabolized protein antigens with the following development of allergic disorders (24). Also, other studies found that patients infected with G. lamblia had high titers of total and specific IgE, as well as cutaneous hyperreactivity for environmental antigens (25, 26). On the other hand, studies found no association between G. lamblia infection and the respiratory tract allergies (27).
5.1. Conclusions
Our study revealed that infections with Ascaris, Toxocara, and Giardia were more common among AR children compared to healthy children and they were significantly associated with the disease severity so the infection with these parasites may be a risk factor for allergic rhinitis among Upper Egyptian children.
5.2. Limitations to the Present Study
There were some limitations to our study. Firstly, it was a case-control study; so, our data did not deliver direct information as to whether parasitic infections are a cause of the development of AR. In addition, we could not do skin prick tests, which helps in the diagnosis of AR.