1. Background
Newborns with small for gestational age (SGA) or large for gestational age (LGA) are seen in approximately 20% of births (1). Increased or decreased intrauterine growth is associated with adverse perinatal outcome and metabolic disturbances in later periods of the life (2). In particular, obesity and obesity related diseases are seen in both newborns with SGA and LGA throughout their lives. Newborns with SGA bear the risk of metabolic disease, especially insulin resistance, glucose intolerance, cardiovascular disease and dyslipidemia (3), on the other hand newborns with LGA also bear the risk of impaired glucose metabolism and cardiovascular disease in the later stages of life (2, 4).
Adipose tissue plays a role in the effects of autocrine, paracrine or endocrine hormons secreting various factors including adipokines as well as being an energy storage organ. In addition, adipose tissue is associated with insulin resistance, obesity and diabetes mellitus (5). In obese diabetic patients, excessive fat tissue has been found to be associated with insulin resistance, dyslipidemia, hypertension, endothelial dysfunction and proinflammatory process. Cytokines / adipokines secreted by adipose tissue play a role in those effects (6, 7).
Omentin-1 (intestinal lactoferrin receptor, intelectin-1, galactofuranose binding lectin, endothelial lectin HL-1), which is an adipokine that weighs 34-kD protein, is secreted from the omental adipose tissue and found at higher concentrations in plasma (8). Omentin-1 involves in a number of physiological events, such as insulin effect, cardiovascular function, and inflammatory response (9). Many studies concerning obesity reported a relationship between metabolic disorders such as insulin resistance, impaired glucose tolerance and type-2 diabetes mellitus, and low omentin-1 values (10, 11).
The amount of adipose tissue is associated with the fetal nutritional status during fetal development (12, 13). This results in adipokine dysregulation throughout fetal life. Also, the adipokine synthesis in placenta and change in the maternal body mass index during pregnancy lead to a modification in the production of various adipokines (14). Many adipokines, such as omentin-1 are released from adipose tissue.
Omentin-1 increases insulin-mediated glucose uptake in adipocytes. Akt is a serine/threonine protein kinase that plays a role as secondary messenger in many important cellular events, such as glucose metabolism, cell proliferation and apoptosis. Omentin-1 increases Akt phosphorylation independently of the insulin. It was reported that omentin performs an insulin signal transduction by activating Akt/protein kinase B and increasing an insulin-stimulated glucose transport (8).
2. Objectives
In the direction of this information, the aim of our study was to compare the omentin-1 values of newborns who were SGA, appropriate for gestational age (AGA) and LGA in the cord blood.
3. Methods
3.1. Patient Selection
This prospective, cross-sectional study was carried out with the measurement of omentin-1 values by Enzyme-linked Immunosorbent method in the cord blood of the infants who were born at the delivery room of Ministry of Health, Istanbul Okmeydani Training and Research Hospital between 01/08/2016 and 31/01/2017. Health Sciences University, Istanbul Okmeydani Education and Research Hospital Ethics Committee approved the study and numbered with 429 on 23/02/2016. The patients who were estimated to comply with the inclusion criteria were informed by the researchers about the study. After obtaining informed consent form from the volunteers for participating in the study, the detailed history of participants was obtained. Gestational age was calculated taking into account the first day of the last menstrual period of mothers.
The AGA group consisted of newborns weighing between 10th and 89th percentiles; The SGA group consisted of newborns whose birth weight was less than 10th percentile and less than 2500 g. The LGA group consisted of newborns whose birth weight was above 89th percentile and > 4000g. Infants, whose mothers were healthy and had no disease; with minimum Apgar scores 7 at 1st and 5th minutes, were included in the study. Newborns who had a poor overall condition, an intrauterine pathology, Apgar scores below 8 at 1st and 5th minutes, a genetic disease, or if mothers had body mass index > 30 kg/m2, gestational diabetes mellitus, any infection, a chronic illness, a history of alcohol or smoking and pregnancy without any follow-up, were excluded from the study. Socio-demographic features and characteristics of mothers as well as the demographic data, weight, gender, and cord blood omentin-1 values of newborns were recorded.
After birth, the mixed arteriovenous cord blood was taken into tubes and immediately centrifuged after coagulation. Supernatant serum was stored at -80°C until the study day. The values of omentin-1 were measured with Enzyme-linked Immunosorbent Assay (Omentin-1 Human, Uscn Life Science Inc., Wuhan, China). The analytical measurement range of Omentin-1 kit was 15.6 - 1000 pg/mL. The limit for low value was 6.2 pg/mL and the average recovery percentage was 93%. The intraassay and interassay variation coefficients were reported to be < 10% and < 12%, respectively. The homeostatic model of insulin resistance assessment (HOMA-IR) was determined with the formula:
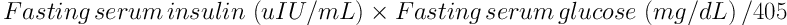
3.2. Statistical Analyses
Statistical analyses were performed with the MedCalc (MedCalc Software, Broekstraat, Mariakerke, Belgium) program. Variables were defined as mean ± standard deviation. The distribution of continuous variables was investigated by the Kolmogorov-Smirnov test. Variants exhibiting Gaussian distribution were indicated as mean ± SD; on the other hand variants exhibiting non-Gaussian distributions were indicated as median (25th percentile - 75th percentile). The mean measure of central tendency was indicated as the confidence interval of 95%. One-way ANOVA analysis was used for the intergroup comparison of variables within normal distribution. Tukey HSD or Tamhane's T2 tests were used in the post hoc comparison. The chi-square test was used to compare group ratios. Correlations between variables were examined with Spearman correlation coefficient (rs) or with Pearson's correlation coefficient (r). Statistical significance was evaluated at the level of P < 0.05 (two-tailed).
4. Results
In study, omentin-1 values of 30 newborns with AGA, 25 newborns with LGA and 20 newborns with SGA were analyzed. The mean age of mothers of infants with AGA was 26.7 ± 5.7 years; followed by 29.3 ± 6.1 years of mothers who had infants with LGA; and 29.3 ± 5.9 years of mothers who had infants with SGA. The averages of age were found to be statistically similar. Gestational age mean of infants with AGA was 39.4 ± 0.6 weeks; infants with LGA infants were 39.5 ± 0.8 weeks, infants with SGA were 37.8 ± 1.7 weeks. The average of the weight of infants with AGA was 3338.4 ± 384.2 g, infants with LGA were 4207.2 ± 181.6 g, infants with SGA were 2150.8 ± 248.6 g. The gestational age and weight of infants with SGA were statistically found to be significantly lower than those of the other infants. The mean weight, multiparous-primiparous status of mothers, education level, type of birth (normal birth or cesarean section) and TŁ-based monthly family income of three groups were found to be statistically similar (P > 0.05; Table 1).
| Characteristics | AGA (N = 30) | LGA (N = 25) | SGA (N = 20) | P Value |
|---|---|---|---|---|
| Maternal age, y | 26.7 ± 5.7 | 29.3 ± 6.1 | 29.3 ± 5.9 | 0.1870 |
| Maternal weight, kg | 79.4 ± 9.4 | 81.1 ± 10.1 | 78.7 ± 9.2 | 0.6720 |
| Primiparous/multiparous, n/n | 21/9 | 18/7 | 14/6 | 0.6120 |
| Delivery method (normal birth/Cesarean section) | 22/8 | 17/8 | 13/7 | 0.8090 |
| Gestational week | 39.4 ± 0.6a | 39.5 ± 0.8a | 37.8 ± 1.7 | < 0.0001 |
| Educational status, No. (%) | 0.1370 | |||
| NA | 2 (6.7) | 0 (0) | 0 (0) | |
| Primary school | 11 (36.7) | 7 (28.0) | 8 (40.0) | |
| Middle school | 10 (33.3) | 13 (52.0) | 7 (35.0) | |
| High school | 4 (13.3) | 4 (16.0) | 2 (10.0) | |
| University | 3 (10.0) | 1 (4.0) | 3 (15.0) | |
| Monthly income level (TL) | 1728 ± 784 | 1928 ± 581 | 2138 ± 500 | 0.0990 |
Abbreviation: HOMA-IR, homeostatic model of assessment- insulin resistance.
a Tamhane's T2 or Tukey test, P < 0.001 vs. Group SGA.
The mean omentin-1 values of newborns were 344.0 ± 108.4 pg/mL in the AGA group; followed by 293.1 ± 78.1 pg/mL in the LGA group; and 238.9 ± 56.3 pg/mL in the SGA group. The mean omentin-1 value of the SGA group was found to be significantly lower than that of the AGA group. However, a statistically significant difference between omentin-1 values of other groups, was not found (P > 0.05). The glucose, urea, creatinine, insulin and HOMA-IR values of three groups in the cord blood samples were statistically similar (P > 0.05; Table 2).
| Variables | AGA (N = 30) | LGA (N = 25) | SGA (N = 20) | P Value |
|---|---|---|---|---|
| Infant weight, g | 3338.4 ± 384.2 | 4207.2 ± 181.6a | 2150.8 ± 248.6a,b | < 0.0001 |
| Baby gender, M/F | 13/17 | 14/11 | 11/9 | 0.5830 |
| Cord blood omentin-1, pg/mL | 344.0 ± 108.4 | 293.1 ± 78.1 | 238.9 ± 56.3a | < 0.0001 |
| Glucose, mg/dL | 92.37 ± 8.8 | 95.13 ± 7.6 | 91.9 ± 6.7 | 0.143 |
| Urea, mg/dL | 14.13 ± 4.4 | 12.03 ± 4.5 | 13.03 ± 4.7 | 0.212 |
| Creatinin, mg/dL | 0.72 ± 0.16 | 0.74 ± 0.21 | 0.76 ± 0.14 | 0.673 |
| Insulin, µIU/mL | 7.73 ± 3.1 | 9.9 ± 3.4 | 8 ± 3.60 | 0.543 |
| HOMA-IR | 1.7 ± 0.7 | 2 ± 0.8 | 1.8 ± 0.8 | 0.512 |
Abbreviation: HOMA-IR, homeostatic model of assessment- insulin resistance.
a Tamhane's T2 or Tukey test, P < 0.0001 vs. AGA group.
b Tamhane's T2 or Tukey test, P < 0.0001 vs. LGA group.
There was no statistically significant relationship between the omentin-1 values of newborns and characteristics of mothers (P > 0.05; Table 3).
| AGA (N = 30) | LGA (N = 25) | SGA (N = 20) | |
|---|---|---|---|
| Maternal age | |||
| r | -0.065 | -0.052 | -0.055 |
| P | 0.731 | 0.805 | 0.818 |
| Maternal weight | |||
| r | -0.177 | -0.073 | -0.099 |
| P | 0.351 | 0.727 | 0.678 |
| Primiparous/multiparous | |||
| rs | 0.357 | -0.131 | -0.045 |
| P | 0.053 | 0.533 | 0.849 |
| Delivery method | |||
| rs | -0.395 | 0.378 | |
| P | 0.050 | 0.100 | |
| Gestational week | |||
| r | 0.257 | 0.183 | 0.119 |
| P | 0.171 | 0.381 | 0.618 |
| Monthly income level | |||
| r | -0.020 | 0.189 | -0.422 |
| P | 0.918 | 0.367 | 0.064 |
| Infant weight | |||
| r | -0.158 | 0.050 | 0.274 |
| P | 0.404 | 0.814 | 0.242 |
| Infant gender | |||
| rs | 0.082 | -0.212 | 0.078 |
| P | 0.668 | 0.308 | 0.742 |
5. Discussion
The lower omentin-1 values of SGA newborns compared to that of other newborns suggests that there is a relationship between omentin-1 values and low birth weight and fetal development. There was no study to compare with our findings, so omentin-1 levels in the SGA group were evaluated for the first time in our study. Decreased serum omentin-1 levels in patients with impaired glucose metabolism cause an insulin-induced glucose uptake into visceral adipocytes or other insulin-sensitive tissues (8). Therefore, the influence of insulin decreases if there is an omentin-1 deficiency. It has been reported that the insulin secretion by the fetal pancreas regulates the fetal growth by maternal glucose concentrations (15). Pancreatic agenesis, which causes the absence of fetal insulin secretion, leads to a decrease in body weight (16). In animal models, hyperleptinemia and hyperinsulinaemia were seen in animals with leptin and insulin resistance. The growth retardation was reported to be the key element for the development of hyperphagia, obesity and hypertension (17). Insulin is essential for growth and the lack of insulin causes weight loss and other complications. Lower levels of omentin-1 in newborns with SGA are likely to reduce insulin effects, thus leads to a decreased fetal growth and low birth weight (12, 13).
It is well known that newborns with SGA bear the risk of obesity and an altered body composition in postnatal growt related to unfavorable intrauterine conditions. Decreased insulin secretion and increased insulin resistance cause the development of metabolic disease in adolescent and adult ages (18). It was reported that patients with impaired glucose tolerance or type-2 diabetes mellitus had increased serum glucose levels and insulin levels as well as decreased omentin-1 levels compared to the normal population (17). Omentin-1 levels were reported to be associated with an increase in serum glucose or insulin levels. Decreased levels of omentin-1 may increase insulin resistance and insulin levels (11, 19, 20). It is not known whether there are other factors affecting omentin-1 levels and the low omentin-1 levels result in increasing glucose and insulin levels yet.
Kafalidis et al. evaluated the umbilical cord omentin-1 values of newborns with LGA and AGA and found that omentin-1 levels of newborns with LGA were significantly higher compared to newborns with AGA. However, they found that omentin-1 levels were significantly lower in vaginal deliveries compared to the control group (2). The results of our study were not compared with those in this study, because omentin-1 levels were determined at different levels according to the birth pattern and gestational diabetes mellitus was reported in 16 of 61 mothers. Newborns with LGA have more adipose tissue and more released omentin-1. However, omentin values of newborns with LGA and AGA were statistically similar. Increased insulin secretions in newborns with LGA were likely to depress omentin-1 secretion. Insulin levels remain to be higher in newborns with LGA without an insulin resistance (2). But, insulin values of newborns with SGA, LGA, and AGA were statistically similar. Other factors, such as a low-calorie diet, high glucose und insulin intake, might affect omentin-1 values as well (21). Lower levels of omentin-1 may be an important factor in the development of postnatal complications such as obesity in newborns with SGA. Lower omentin-1 levels in newborns with SGA could be beneficial in the early detection of obesity and related complications. The exercise, diet, or medical regimens could prevent the development of related complications.
We also examined the socioeconomic status of pregnant women in our study. However, we could not find any difference in terms of educational status and income level between the groups. That might be related to the fact that our study was conducted in a public hospital where people having similar social status often get health care.
5.1. Conclusions
We have determined serum omentin-1 levels to be lower in infants with SGA. The follow-up of infants with SGA and low omentin-1 values might be beneficial in terms of metabolic complications. It is obvious that there is a need for prospective studies including greater number of cases to assess the relationship between omentin-1 levels and methabolic complications.
