1. Background
Every year approximately 2.2% - 16 % of children are born small for gestational age (SGA). It is about 400,000 newborns yearly in the United States (1). In addition, the incidence of SGA in very preterm infants is estimated to encompass 15% to 30% (2). Small fetal size is known to be a risk factor for perinatal morbidity and mortality (3), but is also associated with adverse short and long-term consequences (4). There are research trials showing reduced brain volumes and alterations in brain structure in intra uterine growth restriction (IUGR) premature children compared to controls (5).
However, there are few publications assessing the impact of SGA on later neurodevelopment of premature infants. There are studies showing no difference in long term neurodevelopmental outcomes in SGA preterm (6). Some publications indicate that only girls are at increased risk of neurodevelopmental impairment (7), whereas others report higher risk of cognitive and behavioral difficulties (8-10) and increased autism spectrum disorder frequency (11, 12). Furthermore, AGA controls often differed in gestational age, other birth parameters or frequency of prematurity complications. This fact makes it unclear whether the neurological and cognitive impairment of SGA group was only due to impaired prenatal growth.
2. Objectives
The aim of our study was multifaceted neurodevelopmental evaluation of prematurely born children, with particular assessment of SGA as an independent risk factor for impairment in prematurely born children.
3. Methods
The study was planned as a follow-up of a project conducted between June 2008 and April 2011 in University Children’s Hospital in Cracow. The main aim of the study was to evaluate the role of biochemical and genetic factors in the development of late complications of prematurity. Children who survived were invited to participate in the follow-up study at the chronologic age of 4 years (n = 101). All children were born prematurely with birth weight below 1500 grams. Those with severe congenital health problems (brain malformations, chromosome aberrations, multiple congenital malformations) were excluded from the study. Overall assessment of the group’s neurodevelopment is already published (13). This article concentrates on SGA as risk factor for children’s impairment. The studied children were divided into two groups: appropriate for gestational age (AGA) and small for gestational age (SGA) group. The SGA was defined as birth weight below 10 percentile according to gestational age of the child. Gestational age was defined on the basis of early ultrasound (available in 69% children) and on the last menstrual period supported by Balard examination (in all cases results were comparable). SGA group was divided in two subgroups: symmetric (head circumference below 10th centile) and asymmetric (head circumference above 10th centile).
The study was conducted in the Pediatric Follow-up Department (Department of Pediatrics, Jagiellonian University, Cracow). The patients were recruited between September 2012 and April 2015. All parents signed informed consent. Anthropometric measurements and psychomotor development evaluation were performed in all participants. Parents were asked to complete questionnaires assessing socio-economic status of the family (place of living, parent’s educational status and employment, siblings, children attendance in nursery and kindergarten). The study was approved by the Ethics Committee of Jagiellonian University, Faculty of Medicine.
3.1. Anthropometric Measurements
Body weight and height were measured to the nearest 0.1 kg and 0.1 cm. Head, waist and arm circumference were measured to the nearest 0.1 cm. Original data was converted to z-scores. Catch-up growth was defined as weight, height and head circumference greater than or equal to -2SD of World Health Organization reference values.
3.2. Psychomotor Development
For neurodevelopmental examination the following examination tools were used:
3.2.1. WeeFIM Scale (Functional Independence Measure Scale)
WeeFIM scale is used in measurement and assessment of functional independence of preschool children in their home and environment. Questionnaire is filled in by physician basing on parents’ answers about the level of their assistance in children’s everyday activities. It is designed for patients aged from 6 months up to 8 years. It is a clinically useful, brief, uniform functional disability test. Msall ME et al. (14) recommend WeeFIM for children between 2 and 5 years as a simplest test for patients (15-17). The use of the WeeFIM® instrument to collect data for this research study was authorized and conducted in accordance with the terms of a special purpose license granted to Licensee by Uniform Data System for Medical Rehabilitation (a division of UB Foundation Activities, Inc., “UDSMR”). Licensee has not been trained by UDSMR in the use of the WeeFIM® instrument, and the patient data collected during the course of this research study has not been submitted to or processed by UDSMR. No implication is intended that such data has been or will be subjected to UDSMR’s standard data processing procedures or that it is otherwise comparable to data processed by UDSMR.
3.2.2. Leiter Scale
Leiter scale is a non-verbal psychometric evaluation for children from 3 to 15 years of age. It is a measure of nonverbal intelligence which includes diverse aspects of cognition, ability to solve novel problems (that are not culturally determined or tied to “school learning”) and basic level of visual ability. It is an individual test and tasks are very interesting for children (18). It was the only test standardized for 4-year old children available in Poland at the time of the study.
3.2.3. Developmental Test of Visual Perception
Visual perceptive abilities were assessed with the use of the most recent polish version of the classic Marianne Frostig DTVP (19). The test consists of five subtests (Eye-Motor Coordination test, Figure-Ground test, Consistancy of Shape test, Position in Space test, Spacial Relationships test). DTVP has been validated and proved to be internally consistent, comparing to other tools assessing visual perception, such as Beery-Buktenica Developmental Test of Visual-Motor Integration (VMI) and Test of Visual Perceptual Skills (TVPS-3) (20). The results provide insight into child’s general visual perceptual abilities as well as indicate specific strengths and weaknesses.
3.2.4. The Childhood Autism Spectrum Test
The Childhood Autism Spectrum test (CAST) (21) is used for assessing the severity of autism spectrum symptoms in children. It is designed for children aged 4 to 11 years old. Questionnaire is a 39-item, yes or no evaluation aimed at parents or child’s legal guardian. Maximum score is 31 points. Scores in the 0 - 11 range indicate little or no autistic traits. Score from 12 to 15 may indicate some autism spectrum disorder presence. Scores > 15 indicate high risk of autism diagnosis.
3.2.5. EAS-C
EAS-C is a questionnaire used to assess the temperament, understood as a combination of inherited personality traits. The Buss and Plomin behavior-genetic theory of temperament is the theoretical basis of this tool. The versions for children refers to observational data obtained from parents and teachers about child's shyness, sociability, activity and emotionality.
3.2.6. Children’s Vocabulary Test
Children’s Vocabulary test (TSD) (22) is designed to measure verbal ability, both in terms of producing and understanding language. In addition to the overall score, two specific indicators are obtained from the test: passive and active speech score. It is designed for children aged 4 - 7 years, and is the only vocabulary test available in Poland, culturally appropriate for the target population. The children’s vocabulary test consists of four subtests: two of them measure passive speech and two-active speech. Tasks are given orally to the child and answer sheet is completed by a person conducting the test.
3.3. Outcome Variables
Primary outcomes were defined as:
1) The diagnosis of cerebral palsy;
2) The result of WeeFIM test below 85% of predicted value;
3) The result of Leiter test below 85 points;
4) The results of DVPT-2 (Frostig test) below 85 points;
5) The difference in autism spectrum disorder frequency.
Secondary outcome variables were absolute results of neurodevelopmental tests (WeeFiM, Leiter, DVPT-2 Frostig, EAC-S, Vocabulary/speech test).
3.4. Statistical Analysis
Statistical analysis was performed with the use of Statistica 10.0 software. To assume the differences in continuous variables between studied groups Student’s t-test and Mann-Whitney test were used. Qualitative values were compared by Fischer exact test and Pearson’s chi-Square test. Differences were found as statistically important if the probability of type I error was lower than 0.05.
4. Results
4.1. Group Characteristics
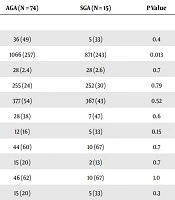
Eighty-nine children (41 girls, 48 boys) born prematurely (mean gestational age 27.8 ± 2.4 weeks) were evaluated. In the analyzed group 15 infants were born small for gestational age (mean birth weight 871 ± 243 grams) and 74 with weight appropriate for gestational age (mean birth weight 1066 ± 257). The groups were similar with respect to gestational age, gender and common perinatal morbidities. The frequency of prematurity complications such as high grade intraventricular hemorrhage (IVH), periventricular leukomalacia and retinopathy of prematurity (ROP), was similar in both groups. Comparison of selected demographic and clinical variables between AGA and SGA newborns are presented in Table 1.
| AGA (N = 74) | SGA (N = 15) | P Value | |
|---|---|---|---|
| Demographic variables | |||
| Female | 36 (49) | 5 (33) | 0.4 |
| Birth weight, g | 1066 (257) | 871 (243) | 0.013 |
| Gestational age, week | 28 (2.4) | 28 (2.6) | 0.7 |
| Head circumference, mm | 255 (24) | 252 (30) | 0.79 |
| Length, mm | 377 (54) | 367 (43) | 0.52 |
| Vaginal delivery | 28 (38) | 7 (47) | 0.6 |
| Multiple pregnancy | 12 (16) | 5 (33) | 0.15 |
| Surfactant administration | 44 (60) | 10 (67) | 0.7 |
| PDA treatment | 15 (20) | 2 (13) | 0.7 |
| Oxygen at 28th day of life | 46 (62) | 10 (67) | 1.0 |
| Oxygen at 36 weeks postmenstrual age | 15 (20) | 5 (33) | 0.3 |
| IVH grade III or IV | 10 (13) | 2 (13) | 1.0 |
| PVL | 8 (11) | 4 (27) | 0.11 |
| ROP | 21 (28) | 6 (40) | 0.4 |
| Socioeconomic variables | |||
| Rural residence | 47 (64) | 9 (60) | 1.0 |
| Maternal education (low/middle/high) | 30/27/17 | 7/3/5 | 0.6 |
| Father’s education (low/middle/high) | 34/28/12 | 6/7/2 | 0.8 |
| Non-working mother | 53 (72) | 12 (80) | 0.75 |
| Non-working father | 4 (5) | 1 (7) | 1.0 |
| Sibling at home | 52 (70) | 9 (60) | 0.7 |
| Breast milk feeding | 33 (45) | 2 (13) | 0.02 |
| Kindergarten | 38 (51) | 5 (33) | 0.2 |
| Rehabilitation care | 60 (83) | 10 (67) | 0.14 |
| Anthropometric measurements | |||
| Age at evaluation, y | 4.16 (0.48) | 4.16 (0.38) | 0.97 |
| Height, cm | 101 (4.9) | 101 (5.6) | 0.55 |
| Height (z-score) | -0.15 (1.1) | -0.4 (1.3) | 0.42 |
| Weight, kg | 14.9 (5.3) | 14.7 (3.4) | 0.81 |
| Weight (z-score) | -0.86 (1.2) | -1.1 (1.7) | 0.52 |
| Head circumference, cm | 49.5 (1.9) | 48.5 (2.2) | 0.07 |
| Head circumference (z-score) | -1.6 (1.3) | -2.5 (1.6) | 0.03 |
| Waist circumference, cm | 49.4 (8) | 48 (4.9) | 0.51 |
| Arm circumference, cm | 16.1 (1.5) | 16.1 (2.1) | 1.0 |
| Arm circumference (z-score) | -0.21 (1.1) | -0.19 (1.5) | 0.96 |
aValues are expressed as No. (%)or mean (SD).
Mean age at the time of neurodevelopmental evaluation was 50 months and was identical in both subgroups. Apart from lower percentage of SGAs fed with breast milk after birth (13% vs 45%; P = 0.02) there were no differences in socioeconomic parameters between groups, including attendance at kindergarten and rehabilitation requirement. According to anthropometric parameters, SGA group had lower head circumference (SDS = -2.5 vs -1.6; P = 0.03) at the age of 4 years. There were no other differences between groups. The comparison of selected socioeconomic variables between the groups and anthropometric measurements in 4th year of life are shown in Table 1.
4.2. Neurodevelopmental Assessment
4.2.1. Cerebral Palsy
In regard to CP incidence, 6 out of 74 (8%) patients developed CP in the AGA group, whereas in the SGA group it was 1 out of 15 (7%). The difference between groups was statistically insignificant. Main risk factor for CP was high grade IVH (27% of children with severe IVH and 6.7% of those without severe IVH suffered CP; P = 0.028). The incidence of this prematurity complication was exactly the same in AGA and SGA group (13% vs 13%; P = 1).
4.2.2. WeeFIM
In self-reliance measurement, decrease in WeeFIM test results (below 85%) was observed in 12% of AGA and 20% of SGA (not statistically relevant). However, detailed analysis of raw scores of WeeFIM test showed significant differences between compared groups. In autonomy score the average difference between SGA and AGA groups was 10 points (89 ± 20 points in SGA group vs 99 ± 15 points in AGA group; P = 0.034). Similarly, the assessment of WeeFIM test results in percent’s of norm showed 10% difference between SGA and AGA group (100% vs 90%; P = 0.043).
4.2.3. Leiter Test
Birth weight small for gestational age seems to be a risk factor for decrease in Leiter score. 64% of SGA and 10% of AGA children showed significant (< 85 points) decrease in non-verbal intelligence test (P < 0.001). Mean score in Leiter test in SGA group was 87 ± 18 points and in AGA children 100 ± 18 (P = 0.022).
4.2.4. Frostig Test
Low visual perception (Frostig test results < 85 points) was shown in 54% of SGA and 26% of children from AGA group (P = 0.046). In visual perception test, children born with weight small for gestational age achieved lower scores (81 ± 17 points in SGA group vs 93 ± 17 points in AGA group; P = 0.035).
4.2.5. CAST
Autism spectrum disorder (ASD) assessment was performed in 14 SGA and 72 AGA children. Two children (2.3%) received score above 15 points (both were SGA). Three peers scored between 12 and 15 points (one from SGA and two from AGA group). The incidence of ASD was significantly higher in the SGA group (21% vs 2.8%; P = 0.029).
Primary and secondary outcome variables in both groups are presented in Table 2.
| Primary Results | AGA (N = 74) | SGA (N = 15) | P Value |
|---|---|---|---|
| Cerebral palsy | 6 (8) | 1 (7) | 1.0 |
| WeeFiM test < 85% | 9 (12) | 3 (20) | 0.4 |
| Leiter test < 85 points | 7 (10) | 9 (64) | < 0.001 |
| Frostig test < 85 points | 18 (26) | 7 (54) | 0.046 |
| CAST > 11 points | 2 (2.8) | 3 (21) | 0.029 |
| Secondary results | |||
| WeeFiM test (points) | 99 (15) | 89 (20) | 0.034 |
| WeeFiM test, % | 100 (16) | 90 (21) | 0.043 |
| Leiter test (points) | 100 (18) | 87 (18) | 0.022 |
| Frostig test (points) | 93 (17) | 81 (17) | 0.035 |
aValues are expressed as No. (%).
4.2.6. TSD, Vocabulary Test
Active speech score below average was shown in 38% of preterms from SGA group and 31% of children from AGA group (P = 0.3). In passive speech 38% of SGA children and 32% of AGA children had results below average (P = 0.5). Deficits in both active and passive speech was shown in 16% of AGA and 30% of SGA children. Problems with at least one of vocabulary tests was shown in 47% of AGA and 46% of SGA preterms. The z-scores distribution in both groups was comparable and the results are presented in Table 3.
| Vocabulary test | |||
|---|---|---|---|
| AGA (N = 70) | SGA (N = 13) | P Value | |
| Active speech | 0.3 | ||
| Very low (≤ 2SD) | 10 (14) | 2 (15) | |
| Low (-2SD to -1SD) | 12 (17) | 3 (23) | |
| Average (-1SD to 1SD) | 42 (60) | 8 (62) | |
| Good (1SD to 2SD) | 5 (7) | 0 | |
| Very good (> 2SD) | 1 (2) | 0 | |
| Reactive speech | 0.5 | ||
| Very low (≤ 2SD) | 6 (9) | 2 (15) | |
| Low (-2SD to -1SD) | 16 (23) | 3 (23) | |
| Average (-1SD to 1SD) | 44 (63) | 7 (54) | |
| Good (1SD to 2SD) | 4 (6) | 1 (8) | |
| Very good (> 2SD) | 0 | 0 | |
| EAS-C test | |||
| AGA (N = 72) | SGA (N = 14) | P Values | |
| High emotionality | 7 (10) | 2 (14) | 0.63 |
| Hyperactivity | 15 (21) | 6 (43) | 0.096 |
| Low sociability | 10 (14) | 2 (14) | 1.0 |
| Shyness | 11 (15) | 2 (14) | 1.0 |
aValues are expressed as No. (%).
4.2.7. EAS-C
Forty-one prematurely born children had at least one temper problem (48% of target population). However, the temper test showed no statistically important differences between AGA and SGA group. Children born from both groups had similar frequency of high emotionality (10% in AGA vs 14% in SGA group), hyperactivity (21% in AGA vs 43% in SGA group; P = 0.096), low sociability (10% in AGA vs 2% in SGA group) and shyness (11% in AGA vs 2% in SGA group). Exact results of EAS-C tests are presented in Table 3.
4.3. Symmetric and Asymmetric SGA
The group of children born with birth weight small for gestational age was divided into two subgroups, due to criteria of head circumference: symmetric SGA (SYM; head circumference > 10th centile) and asymmetric (aSYM; head circumference ≤ 10th centile). 8 children (53%) were born with symmetric SGA and 7 children (47%) with asymmetric SGA. There were no statistically important differences between subgroups birth parameters (gestational age, gender, perinatal morbidities, and prematurity complications).
According to anthropometric parameters in the 4th year of life, children born with SYM were significantly lighter (z-score -1.7 vs -0.43; P = 0.02) and had smaller head circumference (z-score = -3.4 vs -1.4; P = 0.003). Moreover, symmetric SGA infants presented poorer catch-up growth (12.5% vs 62.5%; P = 0.02). There were no statistically important differences between demographic, clinical and socioeconomic variables between groups. Selected birth parameters and anthropometric measurements at the age of 4 are presented in Table 4.
| Symetric SGA (N = 8) | Asymetric SGA (N = 7) | P Value | |
|---|---|---|---|
| Birth parameters | |||
| Birth weight, g | 919 (225) | 816 (269) | 0.33 |
| Gestational age, week | 29 (2.3) | 27 (2.6) | 0.25 |
| Length, mm | 387 (38) | 347 (40) | 0.13 |
| Head circumference, mm | 239 (29) | 266 (26) | 0.08 |
| Follow-up at 4th year of life | |||
| Height, cm | 991 (48) | 1011 (66) | 0.77 |
| Height (z-score) | -0.65 (1.5) | -0.13 (1.3) | 0.8 |
| Weight, kg | 13.6 (2.4) | 16 (4) | 0.8 |
| Weight (z-score) | -1.7 (1.6) | -0.43 (1.9) | 0.02 |
| Head circumference, cm | 47 (2.3) | 50 (1.2) | 0.003 |
| Head circumference (z-score) | -3.4 (1.6) | -1.4 (0.8) | 0.003 |
| Waist circumference, cm | 46 (3.7) | 50 (5.3) | 0.18 |
| Arm circumference, cm | 15.6 (2.2) | 16.6 (2.1) | 0.8 |
aValues are expressed as No. (%) or mean (SD).
There were no statistically important differences between SYM and aSYM SGA groups in the frequency of CP diagnosis (1 vs 0; P = 0.33), WeeFIM test results (88 ± 28 vs 91 ± 7 points; P = 0.22), Leiter test results (87 ± 20 vs 87 ± 17; P = 0.9), Frostig test results (84 ± 22 vs 79 ± 14; P = 0.8) or ASD diagnosis (25% in SYM vs 14% in aSYM SGA group; P = 0.6). Primary outcomes of SYM and aSYM SGA preterms are presented in Table 5. There were also no differences in EAS-C and in vocabulary test results between SGA subgroups.
| Symetric SGA (N = 8) | Asymetric SGA (N = 7) | P Value | |
|---|---|---|---|
| Cerebral palsy | 1 (12.5) | 0 (0) | 0.33 |
| WeeFiM test < 85% | 2 (25) | 1 (14) | 0.6 |
| WeeFiM test (points) | 88 (28) | 91 (7) | 0.22 |
| Leiter test < 85 points | 5 (62.5) | 4 (57) | 0.58 |
| Leiter test (points) | 87 (20) | 87 (17) | 0.9 |
| Frostig test < 85 points | 3 (37.5) | 4 (57) | 0.8 |
| Frostig test (points) | 84 (22) | 79 (14) | 0.8 |
| CAST > 11 points | 2 (25) | 1 (14) | 0.6 |
aValues are expressed as No. (%) or mean (SD).
5. Discussion
It seems that birth weight small for gestational age is an additional, independent risk factor for neurodevelopmental delay in children born prematurely.
There are many research trials confirming the impact of SGA on neurodevelopmental delay in children born on time (23, 24). In recent years, studies have concentrated on evaluating the possibility of SGA being an independent risk factor for developmental impairment in premature children. Few studies performed in last decades indicate that preterm infants with IUGR are at highest risk for long-term morbidities, including developmental disabilities such as mental retardation, cerebral palsy and a wide spectrum of behavior disorders and learning disabilities (25-27). Our study did not confirm the higher incidence of cerebral palsy in children born with birth weight small for gestational age, but we illustrated lower neurodevelopmental outcomes in this subgroup of preterms.
SGA children had more cognitive problems, lower visual perception and were less independent in everyday life. Mean decrease in self-reliance assessment in SGA group was 10 points. Moreover, SGA children scored 12 points lower in visual perception assessment. The most explicit difference was shown in the non-verbal intelligence analysis (13 points).
In already published (13), overall assessment of neurodevelopment in this group, we showed that severe complications of prematurity (IVH III or IV grade, moderate and severe bronchopulmonary dysplasia, laser therapy of ROP) are the main risk factors for decrease in WeeFIM test. Taking into account the fact that SGA and AGA group did not differ in frequency of severe prematurity complications, our data confirm that being born with birth weight small for gestational age is an independent risk factor for poorer result in self-reliance tests.
Second issue widely analyzed in recently published research trials is the frequency of behavioral disorders in ex-preterms. In our study group 48% of children suffered from some behavioral irregularities. Distribution of sociability, emotionality and shyness scores did not differ from the distribution of population-determined standards, whereas hyperactivity was significantly more frequently reported as compared with the general population. However, we did not notice any relevant differences between SGA group and other premature infants in terms of frequency of behavioral disorders (57% vs 46%; P = 0.56) or hyperactivity diagnosis (43% of SGA vs 21% of AGA, P = 0.096). We suspect that lack of statistical significance of that difference is caused by the high frequency of this problem in our group of children (24%) and the low number of children in SGA subgroup.
Next important issue is the incidence of ASD in premature population. The studies reporting increased frequency of autism spectrum disorders in children born prematurely are only now emerging. Discovering risk factors for that diagnosis is important mainly due to fact, that the causes of autism and ASD are still unknown. It is believed that both genetic (28) and external factors (modifying the development of the brain, differentiation processes, synaptogenesis and myelination) are important (29). In our study, we illustrated that children born prematurely have higher risk of developing ASD if they are born SGA.
Last issue analyzed in our study was the influence of SYM and aSYM small gestational age on the neurodevelopmental tests results. Our study confirms previous reports that there are no differences in neurodevelopmental screening between SGA SYM and aSYM subgroups. However, we cannot ignore the fact that lower head circumference (observed in SYM SGA group) seems to be the risk factor for poorer neurodevelopmental outcome (13). The small sample size may account for the lack of difference identified and warrant further research in this area.
5.1. Strengths and Limitations
In our opinion, the study has significant value and provides a new insight into the neurodevelopmental problems of children born prematurely with very low birth weight. First of all, the perinatal data in our study comes from prospective and systematic observation and the study group included 89 from 101 VLBW children (88% of available population) hospitalized in NICU in a three-year period. Secondly, our SGA and AGA groups were similar in all birth parameters (gestational age, gender, common perinatal morbidities, and frequency of prematurity complications). Furthermore, the evaluation of preterm neurodevelopment was multifactorial and all tests were performed by trained physicians.
The main limitations of our study are small size of SGA group and uneven distribution of patients in studied subgroups.
5.2. Conclusions
Birth weight small for gestational age seems to be an additional, independent risk factor for neurodevelopmental delay of prematurely born children. There are no differences in neurodevelopmental screening between symmetric and asymmetric small gestational age subgroups, but symmetric SGA is correlated with smaller head circumference in 4th year of life and poorer catch-up growth.