1. Background
Neuroblastoma is the most prevalent tumor in neonates (1). The median age at diagnosis is 19 months, with 50% of patients being younger than two years old and 90% below the age of five years (2). Neuroblastoma is derived from multipotent neural crest cells and displays morphologic and histologic characteristics of peripheral sympathetic nerve tumors (3). The lesion is found in the abdomen in approximately 70% of cases (1). Neuroblastoma is very heterogeneous in terms of its biologic, genetic, morphologic and clinical characteristics (4, 5). Little is known about the etiology of this cancer, although maternal, familial and other genetic factors have been implicated, including amplification of the MYCN proto-oncogene, DNA ploidy and allelic deletions on chromosomes 1p and 11q (6). Surgery, chemotherapy, autologous stem cell transplantation, immunotherapy and radiotherapy, either alone or in combination, have been used in treatment of neuroblastoma (7-9). However, the optimal therapeutic strategy has yet to be established. Furthermore, the mortality rate of neuroblastoma remains high, despite improvements in overall survival (OS) between 1975 - 1979 and 2000 - 2006 (10). Furthermore, the outcomes are highly variable between patients, ranging from spontaneous regression in some to life-threatening progression in others (1, 11). Indeed, there is evidence that most tumors detected by ultrasonography during pregnancy do not need treatment after birth and regress spontaneously (2).
Identifying the factors affecting neuroblastoma progression and developing an accurate method of risk stratification would facilitate clinical decision-making regarding the most appropriate therapy for each patient (for example, supportive therapy if tumor regression is expected but more aggressive treatment if disease progression is predicted). To this end, there has been considerable research into the factors affecting the biology of this tumor. For example, the International Neuroblastoma Pathology Classification (INPC) categorizes tumors according to their constituent neural-type cells (primitive, differentiating neuroblasts and maturing ganglion cells) and Schwann-type cells, which partly reflects the tumor’s embryonic origin. The Children’s Oncology Group (COG) makes use of the INPC classification to categorize patients into different risk categories (7, 8, 12), with more primitive constituent cells predictive of worse outcome (i.e., greater risk of metastasis). Furthermore, the International Neuroblastoma Risk Group (INRG) subdivides patients with neuroblastoma into (very) low-, intermediate-, high- and ultra-high-risk groups based on age at diagnosis, clinical stage, tumor histology, grade of tumor differentiation, MYCN oncogene amplification, 11q deletion and DNA ploidy (1, 5, 13). However, in addition to these factors, it is now recognized that tumor microenvironment may play an important role in the metastatic behavior of cancer (3, 14). This raises the possibility that the characteristics of the tissue in which a neuroblastoma is located might influence tumor progression.
Several decades ago, Pierce and colleagues proposed that malignant embryonal cancer cells are derived from normal stem cells and that their differentiation could potentially be regulated by embryonal fields (15). In view of the similarities between embryo development and tumorigenesis, the microenvironment of an embryonal tumor might be capable of regulating its behavior. If so, this raises the possibility that the characteristics and behavior of a neuroblastoma may differ depending on the embryonic origin of the tissue in which it is located (mesodermal, ectodermal or endodermal).
2. Objectives
We hypothesized that the characteristics and biologic behavior of neuroblastoma might differ depending on the embryonic origin of the tissue in which the tumor is located. Therefore, the aim of this study was to analyze Surveillance, Epidemiology, and End Results (SEER) program data for patients with malignant neuroblastoma and compare tumor characteristics between tissues derived from different germ layers.
3. Methods
3.1. Study Participants
This was a retrospective analysis of data extracted from SEER databases for patients diagnosed with malignant neuroblastoma between 1973 and 2014. The inclusion criteria were a confirmed diagnosis of malignant neuroblastoma between 1973 and 2014; patient age at diagnosis was known; and embryonic origin (mesodermal, ectodermal or endodermal) of the primary lesion site was known. The exclusion criteria were patient alive but survival time since diagnosis not known; only death certificate information available; and only autopsy data available.
3.2. Data Analysis
The composition and statistical methods of the SEER registries are described on the SEER website (http://seer.cancer.gov/registries/terms.html). SEER data were extracted using SEER*STAT 8.3.4 (https://seer.cancer.gov/seerstat/).
Patient information was obtained from the “Incidence - SEER 18 Regs Research Data + Hurricane Katrina Impacted Louisiana Cases, Nov 2016 Sub (1973 - 2014 varying)” database according to the following criteria: [Site and Morphology. ICD-O-3 Hist/behave] = ‘9490/3: Ganglioneuroblastoma’, ‘9500/3: Neuroblastoma, NOS’, ‘9522/3: Olfactory neuroblastoma’. The primary tumor location was determined by International Classification of Diseases for Oncology (ICD-O)-3 codes (http://codes.iarc.fr/). In the analysis, patients were grouped into three cohorts according to the embryonic origin of the tissue in which the primary neuroblastoma lesion was located: endoderm group, mesoderm group or ectoderm group. The following demographic and clinical information were extracted from the SEER database: patient age, patient gender, patient race, tumor histologic type, tumor grade, tumor size, tumor extension, lymph node invasion, tumor metastasis and use of surgery.
Since some data regarding the use of radiotherapy were missing from the above database, the “Incidence-Based Mortality - SEER 18 Regs (Excl Louisiana) Research Data, Nov 2016 Sub (2000 - 2014) <Katrina/Rita Population Adjustment>” database was used to analyze the use of radiotherapy and five-year OS according to the embryonic origin of the primary lesion site. OS was defined as the time from diagnosis to death.
3.3. Statistical Analysis
Data were analyzed using SPSS 19.0 (IBM Corp., Armonk, NY, USA). Categorical variables are expressed as frequencies and percentages and were compared between groups using the chi-squared test. OS was analyzed using the Kaplan-Meier method and log-rank test. P < 0.05 was considered statistically significant.
4. Results
4.1. Baseline Characteristics of the Study Participants
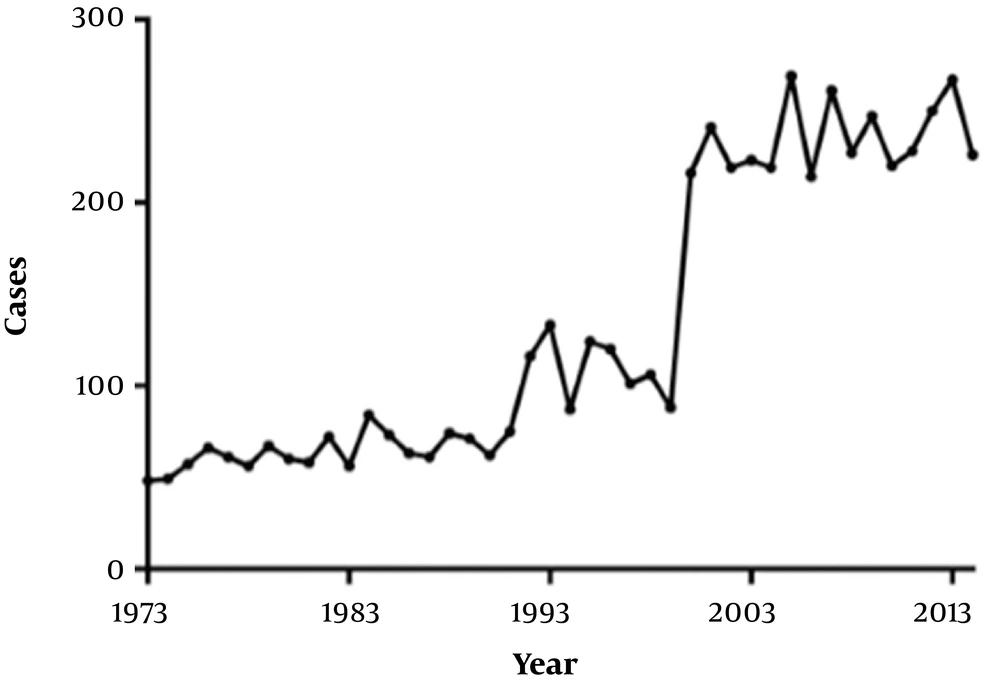
The final analysis included 3701 patients diagnosed with malignant neuroblastoma between 1973 and 2014. The annual number of cases of neuroblastoma in the SEER database increased during the period 1973 - 2014 (Figure 1), although this may partly have been due to an increasing number of SEER registry sites over this period. The baseline characteristics of the study participants are shown in Table 1. Based on the location of the primary lesion, there were 1970 (53.2%) patients in the mesoderm group, 1017 (27.5%) patients in the ectoderm group and 714 (19.3%) patients in the endoderm group. There were no significant differences in patient age, gender or race between the three groups (Table 1). However, the three groups exhibited differences with regard to tumor histology (P < 0.01): ectoderm-derived tissue contained mostly neuroblastoma (79.2%), as did mesoderm-derived tissue (71.1%), whereas endoderm-derived tissue most commonly contained olfactory neuroblastoma (94.7%).
| Variable | Total (N = 3701) | Endoderm (N = 714) | Mesoderm (N = 1970) | Ectoderm (N = 1017) | P Value |
|---|---|---|---|---|---|
| Histology | < 0.001 | ||||
| Neuroblastoma, NOS | 2242 (70.4) | 35 (4.9) | 1401 (71.1) | 806 (79.2) | |
| Ganglioneuroblastoma | 563 (13.5) | 3 (0.4) | 360 (18.3) | 200 (19.7) | |
| Olfactory neuroblastoma | 896 (24.2) | 676 (94.7) | 209 (10.6) | 11 (1.1) | |
| Age, y | 0.373 | ||||
| ≤ 1 | 1596 (43.5) | 292 (40.9) | 880 (44.7) | 424 (41.7) | |
| > 1 and ≤ 2 | 396 (10.7) | 73 (10.2) | 215 (10.9) | 108 (10.6) | |
| > 2 | 1709 (45.7) | 349 (48.9) | 875 (44.4) | 485 (47.7) | |
| Gender | 0.610 | ||||
| Male | 1947 (53.0) | 367 (51.4) | 1048 (53.2) | 532 (52.3) | |
| Female | 1754 (46.8) | 347 (48.6) | 922 (46.8) | 485 (47.7) | |
| Race | 0.421 | ||||
| White | 2906 (78.5) | 542 (75.9) | 1555 (78.9) | 809 (79.5) | |
| Black | 439 (11.9) | 87 (12.2) | 241 (12.3) | 111 (10.9) | |
| Other | 314 (8.5) | 75 (10.5) | 152 (7.7) | 87 (8.6) | |
| Unknown | 42 (1.1) | 10 (1.4) | 22 (1.1) | 10 (1.0) |
aValues are expressed as No. (%).
4.2. Tumor Differentiation and Metastasis
Overall, the tumors were most commonly poorly differentiated, and the mean size was 69.14 ± 58.37 mm (Table 2). Furthermore, most cases showed tumor extension, although lymph node invasion and distant metastases were found in only a minority of cases (Table 2). Based on the data available for the analysis (i.e. excluding cases with missing information), the endoderm group had a higher proportion of well or moderately differentiated tumors (51.4%) than the mesoderm (17.2%) or ectoderm (10.1%) groups as well as significantly smaller tumors (P < 0.05; Table 2). Tumor extension was more common for the endoderm group (72.2%) than for the mesoderm (53.8%) or ectoderm (50.4%) groups (P < 0.05). However, the rates of lymph node invasion and distant metastases were lower for the endoderm group (12.0% and 10.2%, respectively) than for the mesoderm (35.5% and 48.4%, respectively) or ectoderm (40.2% and 48.4%, respectively) groups (P < 0.05).
| Variable | Total (N = 3701) | Endoderm (N = 714) | Mesoderm (N = 1970) | Ectoderm (N = 1017) | P Value |
|---|---|---|---|---|---|
| Grade | < 0.001 | ||||
| Well differentiated, Grade I | 136 (3.7) | 32 (4.5) | 73 (3.7) | 31 (3.0) | |
| Moderately differentiated, Grade II | 193 (5.2) | 133 (18.6) | 51 (2.6) | 9 (0.9) | |
| Poorly differentiated, Grade III | 779 (21.0) | 97 (13.6) | 437 (22.2) | 245 (24.1) | |
| Undifferentiated, Grade IV | 330 (8.9) | 59 (8.3) | 158 (8.0) | 113 (11.1) | |
| Unknown | 2263 (61.1) | 393 (55.0) | 1251 (63.5) | 619 (60.9) | |
| Size (missing data excluded) | < 0.001 | ||||
| N | 1956 | 313 | 1047 | 596 | |
| Tumor size, mm | 69.14 ± 58.37 | 43.89 ± 20.84 | 72.43 ± 67.14 | 63.00 ± 36.98 | |
| Extension | < 0.001 | ||||
| N | 3295 | 682 | 1716 | 897 | |
| Localized | 1074 (29.4) | 171 (23.9) | 553 (28.2) | 350 (35.6) | |
| Further extension | 1444 (39.6) | 444 (62.2) | 644 (32.9) | 356 (36.5) | |
| Unknown | 777 (21.3) | 67 (9.4) | 519 (26.5) | 191 (19.6) | |
| Lymph node invasion | < 0.001 | ||||
| No | 1491 (40.3) | 471 (66.0) | 713 (36.2) | 307 (30.2) | |
| Regional | 602 (16.3) | 59 (8.3) | 354 (18.0) | 189 (18.6) | |
| Distant | 59 (1.6) | 5 (0.7) | 37 (1.9) | 17 (1.7) | |
| Contra/bilateral | 1 (0.0) | 0 | 1 (0.0) | 0 | |
| Unknown | 1548 (41.8) | 179 (25.0) | 865 (43.9) | 504 (49.6) | |
| Distant metastasis | < 0.001 | ||||
| No | 1170 (31.6) | 380 (53.2) | 489 (24.8) | 301 (29.6) | |
| Yes | 784 (21.2) | 43 (6.0) | 459 (23.3) | 282 (27.7) | |
| Unknown | 1747 (47.2) | 291 (40.8) | 1022 (51.9) | 434 (42.7) | |
| Surgery | < 0.001 | ||||
| No | 673 (18.2) | 124 (17.4) | 319 (16.2) | 230 (22.6) | |
| Yes | 2728 (73.7) | 571 (80.0) | 1422 (72.2) | 735 (72.3) | |
| Unknown | 300 (8.1) | 19 (2.6) | 229 (11.6) | 52 (5.1) |
aValues are expressed as No. (%).
4.3. Treatment and Overall Survival
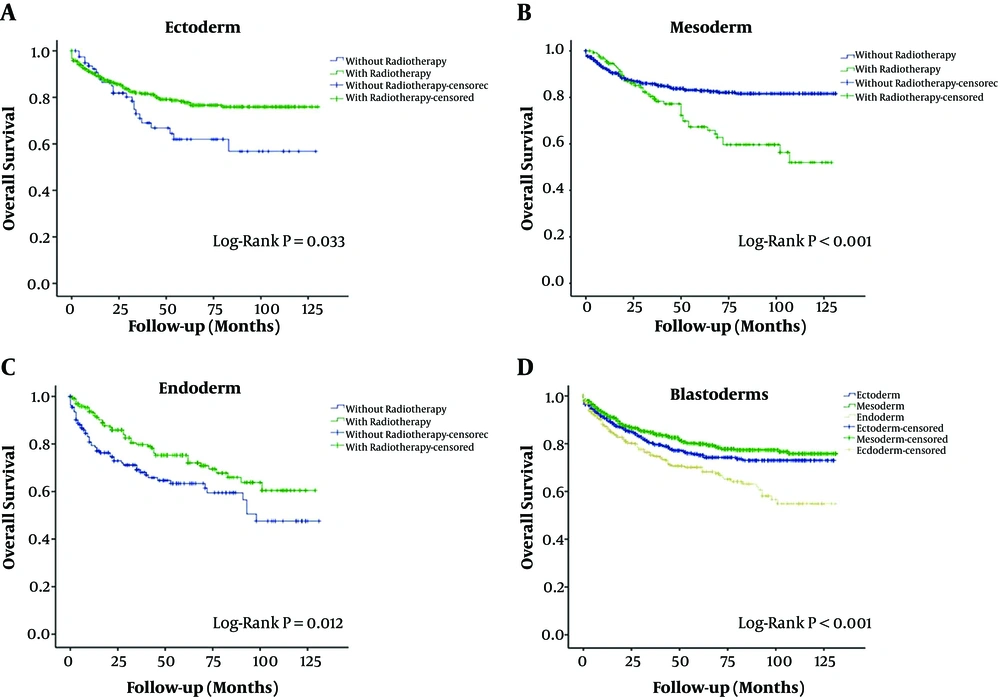
Most of the patients diagnosed with neuroblastoma received surgical treatment (2728, 73.7%). Patients in the endoderm group had poorer overall survival (OS) than those in the other two groups (P < 0.05; Figure 2). Importantly, inclusion of radiotherapy as part of the treatment protocol improved OS in the endoderm and ectoderm groups but worsened OS in the mesoderm group (P < 0.05; Figure 2).
Kaplan-Meier analysis of overall survival. (A) Overall survival in the ectoderm group, comparing patients who received radiotherapy and those who did not (P = 0.033, log-rank test). (B) Overall survival in the mesoderm group, comparing patients who received radiotherapy and those who did not (P < 0.001, log-rank test). (C) Overall survival in the endoderm group, comparing patients who received radiotherapy and those who did not (P = 0.012, log-rank test). (D) Comparison of overall survival between the endoderm, mesoderm and ectoderm groups (P < 0.001, log-rank test).
5. Discussion
As a notable finding of this study, neuroblastoma was found most commonly in mesoderm-derived tissues and least often in endoderm-derived tissues. Furthermore, tumor histology differed between groups, with neuroblastoma most common in the ectoderm and mesoderm groups but olfactory neuroblastoma most common in the endoderm group. Importantly, the endoderm group had smaller and better-differentiated tumors than the other groups as well as a lower prevalence of lymph node invasion and metastasis. However, OS was poorest for the endoderm group. In addition, radiotherapy improved OS in the endoderm and ectoderm groups but worsened survival in the mesoderm group. Taken together, our novel findings suggest that the characteristics and behavior of neuroblastoma can be influenced by the tumor microenvironment, specifically the embryonic origin of the host organ/tissue.
To the best of our knowledge, no previous studies have examined whether the embryonic origin of the primary lesion site affects the behavior of neuroblastoma. Neuroblastoma is derived from neural crest cells, which form part of the ectoderm. An important finding of this analysis was that neuroblastoma and ganglioneuroblastoma were predominantly found in tissues of mesodermal and (less commonly) ectodermal origin, whereas olfactory neuroblastoma was predominantly encountered in endodermal tissue. These observations suggest that the microenvironment of organs derived from mesoderm and, to a lesser extent, ectoderm may be relatively more suitable for the development of neuroblastoma and ganglioneuroblastoma, while the microenvironment of the endoderm may be more favorable for olfactory neuroblastoma. In addition, tumors located in endoderm-derived tissue were generally smaller, better differentiated and less likely to show lymph node invasion or metastasis. This raises the intriguing possibility that the development and progression of neuroblastoma are better controlled in organs originating from the endoderm, as compared with mesoderm or ectoderm. Thus, the microenvironment within the endoderm may be less optimal for the development of neuroblastoma that exhibits aggressive behavior (such as lymph node invasion and distant metastasis). Consistent with this, a preclinical study has suggested that factors derived from the microenvironment of the lung (which is endodermal in origin) can reduce the viability of neuroblastoma cells by regulating the expression of pro-apoptotic genes (16). It may be that the various immune escape mechanisms adopted by neuroblastoma (17) are less effective in tissues of endodermal origin, resulting in better control of tumor growth and progression.
An apparently contradictory observation to the above findings was that patients with tumors in endoderm-derived tissue had poorer OS than patients with neuroblastoma in ectoderm- or mesoderm-derived organs. However, a possible reason for the poorer survival rate in the endoderm group is that the tumors in these patients were mainly found in the respiratory and intestinal systems, which have important physiologic functions that are critical to life. In addition, olfactory neuroblastoma comprised the majority of tumors in the endoderm group but the minority in the other two groups and this may also have contributed to the differences in survival. It is also possible that the three groups showed differences in other factors affecting prognosis, such as MYCN oncogene amplification, 11q deletion and DNA ploidy (1, 5, 13, 18). Although patient age is also considered a prognostic factor in patients with neuroblastoma (1, 5, 13, 18), it did not differ between groups in our study and so is unlikely to be a contributing factor to differences in survival.
Although data are limited, previous studies have suggested that radiotherapy can achieve good local control of neuroblastoma (19) and that the addition of radiotherapy to surgery can slightly improve five-year survival (20), while not all studies have observed a survival benefit (21). In the present study, the inclusion of radiotherapy in the management plan was associated with improved OS in the endoderm and ectoderm groups but worse OS in the mesoderm group. This suggests that neuroblastoma in endoderm- or ectoderm-derived tissue may respond favorably to radiotherapy whereas the adverse effects of radiation may outweigh any benefits when the tumor is in mesoderm-derived tissue. This novel finding warrants further investigation as, if verified, could provide a potential new method of identifying patients most likely to respond to radiotherapy. Irrespective of these findings, we believe that radiotherapy should be used with caution in infants less than two years old due its numerous short- and long-term adverse effects (22), including immune system dysfunction and risk of relapse, that substantially reduce quality of life.
The mechanisms underlying the different characteristics and behavior of neuroblastoma between endoderm-, ectoderm- and mesoderm-derived tissue remain unknown. A variety of factors may be involved in the development and progression of neuroblastoma, including histone demethylation by lysine-specific demethylase (8), intracellular signaling cascades regulated by reactive oxygen species, including extracellular signal-regulated kinase 1/2 (ERK1/2), c-Jun N-terminal kinase (JNK), p38 mitogen-activated protein kinase (p38 MAPK) and protein kinase B (PKB) (9), neuroblastoma breakpoint family (NBPF)-1 (23), the activin-CFC1 signaling axis (24, 25), semaphorin 3C expression level (26) and mutations in genes such as ALK (anaplastic lymphoma kinase), PTPN11 (protein tyrosine phosphatase, non-receptor type 11), ATRX (ATP-dependent helicase), MYCN (N-myc) and NRAS (N-Ras) (27). Future studies are needed to examine whether these and/or other factors contribute to the effects observed in the present investigation.
This study has some limitations. First, this was a retrospective study that only used data from SEER databases, hence the findings may be subject to selection and information bias. Furthermore, the generalizability of the results is not known. Second, records for benign cases of neuroblastoma in the SEER database were lacking, so an analysis of benign neuroblastoma was not undertaken. Third, it was not possible to study the mechanisms underlying the effects observed.
In conclusion, the characteristics and behavior of neuroblastoma are affected by the embryonic origin of the organ/tissue in which the tumor is located. Further research is merited to establish whether the embryonic origin of the tissue can be used as an additional factor for risk stratification of patients with neuroblastoma.