1. Background
Cystic fibrosis (CF) is a progressive, silent, and chronic disease with multiple complications associated with concentrated mucus, malabsorption, and infection. The respiratory epithelium of CF patients shows high permeability to chlorine and high reabsorption for sodium. These changes in epithelium bioelectrical properties lead to relative dehydration of airway secretion and disturbance in mucus membrane transmission and airway obstruction (1, 2). Cystic fibrosis is the most common hereditary congenital anomalous disease in children, characterized by multiple obstructive tract infections and digestive complications (3). These complications include low growth rates and growth impairment. Furthermore, CF can also be counted as a reason for failure to thrive (4-6). Itd incidence is estimated one case in every 1566 births in USA, 3 in 32666 among African-Americans, 3 in 13666 in Asians, and low prevalence was found in other populations (7). Pulmonary infections with certain virulent species, especially bourcheleria sepsis, are difficult to treat and may be associated with clinical worsening of the patient’s condition. Allergic aspergillosis bronchopulmonary may also be a complication of CF disease, and it is necessary to treat cases by steroids and antifungal agents (8, 9). Other potential pulmonary complications of CF include atelectasis, progressive bronchitis, hemoptysis, and pneumothorax. The diagnosis of CF should be taken seriously in any disease with chronic or recurrent respiratory and gastrointestinal symptoms (10). Phenotype features of CF include chronic pulmonary and sinus disease, gastrointestinal and nutritional malfunctions, salt loss syndrome, and obstructive azoospermia (11). The most important factors involved in growth failure among CF patients are undernutrition or malnutrition, chronic inflammation, lung disease, and corticosteroid treatment (12-14). Nutritional support and pharmacological therapy with recombinant human growth hormone are essential for a good management of children with CF, although these children are shorter and lighter than healthy children, and despite the catch-up growth observed after diagnosis, deficit in length/height and weight continues to be seen until adulthood (15-17).
2. Objectives
Considering that children with CF suffer from low growth indices and based on studies published in related literature, the level of growth hormone and associated peptides is reported to be low in these patients (18, 19), and growth indices increase in response to administration of growth hormone (20), we decided to assess the level of growth-related hormones in CF patients, who were referred to Sheikh Hospital of Mashhad, Iran.
3. Methods
3.1. Patients
This was a cross-sectional descriptive-analytic study. Sample size was calculated as 60 cases with a probability accuracy of 0.5 (21). Inclusion criteria were being a child with cystic fibrosis that was confirmed by sweat test and referred to Pediatric Sheikh Hospital. Exclusion criteria included parental refusing of examination and sampling, inability in sampling and child’s poor cooperation, excessive physical weakness of the patient, which could lead to lab data error, and hospitalization because of related CF complications such as pulmonary involvement. Patients with other chronic diseases like hypothyroidism or type 1 diabetes, which might affect the test results, were also excluded.
3.2. Methods
All CF patients in the age range of 7 months to 18 years were enrolled.
Ten cc of blood was taken from the radial vein of each patient by a skilled sampler and GH, IGF1, and IGFBP3 levels were measured. In each visit, the height and weight of patients were examined and recorded by a pediatrician. Weight was measured by the SECA digital scale, appropriate for patient’s age, and height by a flexible tape measure that couldn’t be stretched. Growth failure was defined as z score of weight and height lower than -2. The researchers used the charts of World Health Organization (WHO) (22) to measure growth indices
3.3. Statistical Analysis
The clinical and laboratory data were analyzed by SPSS version 16. Descriptive statistics were presented by mean, medium, and tables. Analytical statistics were analyzed by chi Square and Mann-Whitney tests.
3.4. Ethic Considerations
Necessary information was given to children (when applicable) and their parents about the project and written consents from parents obtained. Responding to possible questions from patients or their parents was available. The researchers ensured them to keep their information confidentially and they had the right to leave the study any time if they didn’t want to continue.
4. Results
4.1. Demographic and Clinical Findings
Totally 60 children, 36 (60%) males and 24 (40%) females, were studied. The Z score of weight was -1.88 ± 1.77 for males and -1.84 ± 2.67 in females (P = 0.948). The Z score for height was -2.11 ± 1.72 for males and -1.93 ± 2.36 for females (P = 0.804). There was no significant difference between male and female patients in scores of weight and height, and the average age was 48 ± 81.9 months. The mean age at the onset of first clinical symptoms of CF was 3.4 ± 1.1 months and the disease was diagnosed at an average age of 11.8 ± 3.09 months. 90.3% of the patients were under one year old when CF was diagnosed and 74.20% showed the first clinical signs of the disease at this age.
The means of body mass index, height, and weight were 15.9 ± 3.2 kg per m2, 116 ± 20 cm, and 22.4 ± 8.8 kg respectively. The most common symptoms at the time blood tests were taken, consisted of steatorrhea (46%), cough (39.3%), and pneumonia (39.3%).
4.2. Experimental Indices
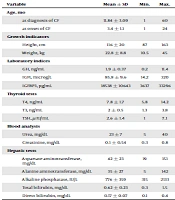
In 20 children, serum level of growth hormone was less than the normal index for their age. Also, insulin-like growth factor (IGF1) was found lower than normal range in 6 (8.8%) patients, and low level of insulin-like growth factor-binding protein 3 (IGFBP3) in 6 (8.8%) cases. The mean serum levels of T3 and T4 were 2 ± 0.5 ng/mL and 7.8 ± 1.7 ng/mL respectively, and mean TSH was 2.6 ± 1.4 micro international units per milliliter. The mean levels of urea and creatinine of patients were 23 ± 7 and 0.54 ± 0.1 mg/dL. The mean total and direct bilirubin in children with CF were 0.62 ± 0.23 and 0.17 ± 0.07 mg/dL, respectively. Also the mean AST, ALT, and ALKP were 42 ± 23, 35 ± 27, and 776 ± 359 micro international units per milliliter (Table 1).
| Variable | Mean ± SD | Min. | Max. |
|---|---|---|---|
| Age, mo | |||
| At diagnosis of CF | 11.84 ± 3.09 | 1 | 60 |
| At onset of CF | 3.4 ± 1.1 | 1 | 24 |
| Growth indicators | |||
| Height, cm | 116 ± 20 | 87 | 163 |
| Weight, kg | 22.8 ± 8.8 | 10.5 | 45 |
| Laboratory indices | |||
| GH, ng/mL | 1.9 ± 0.37 | 0.2 | 11.4 |
| IGF1, microg/L | 85.8 ± 9.6 | 14.2 | 320 |
| IGFBP3, pg/mL | 18538 ± 10643 | 3637 | 33296 |
| Thyroid tests | |||
| T4, ng/mL | 7.8 ± 1.7 | 5.8 | 14.2 |
| T3, ng/mL | 2 ± 0.5 | 1.3 | 3.8 |
| TSH, µIU/mL | 2.6 ± 1.4 | 1 | 7.1 |
| Blood analysis | |||
| Urea, mg/dL | 23 ± 7 | 5 | 40 |
| Creatinine, mg/dL | 0.1 ± 0.54 | 0.3 | 0.8 |
| Hepatic tests | |||
| Aspartate aminotransferase, mg/dL | 42 ± 23 | 19 | 151 |
| Alanine aminotransferase, mg/dL | 35 ± 27 | 5 | 142 |
| Alkaline phosphatase, IU/L | 776 ± 359 | 315 | 2133 |
| Total bilirubin, mg/dL | 0.62 ± 0.23 | 0.3 | 1.5 |
| Direct bilirubin, mg/dL | 0.17 ± 0.07 | 0.1 | 0.4 |
4.3. Mean Serum Level of Hormonal Indices Based on Clinical Signs
Serum level of GH, IGF1, and IGFBP3 were lower in patients with growth failure, however, it was only in IGFBP3 level statically significant (P = 0.004).
The mean level of IGF1 and IGFBP3 in serum of patients with pancreatic failure was lower than other patients, yet this was not statistically significant (P > 0.05).
4.4. Mean Serum Level of Hormonal Indices Based on Age Groups
The mean serum level of Growth Hormone was not significantly different between age groups (P = 0.09). Also, no significant difference was found between age groups in terms of IGF1 (P = 0.136) and IGFBP3 (P = 0.156).
Dividing the patients into two groups of lower and higher than five years old, a significant difference was found for IGF1 between the two groups (P = 0.009) see Table 2.
| Indices | Growth Failure | Mean ± SD | P Value |
|---|---|---|---|
| Mean of serum level | |||
| GH, ng/mL | Yes | 1.2 ± 0.97 | 0.948 |
| No | 2.1 ± 0.87 | ||
| IGF1, microg/L | Yes | 102 ± 85 | 0.695 |
| No | 112 ± 70 | ||
| IGFBP3, pg/mL | Yes | 17125 ± 3674 | 0.004 |
| No | 23425 ± 5178 | ||
| Age, y | |||
| GH, ng/mL | Less than 5 years | 2.2 ± 0.52 | 0.536 |
| Older than 5 years | 1.7 ± 0.53 | ||
| IGF1, microg/L | Less than 5 years | 62 ± 43 | 0.009 |
| Older than 5 years | 109 ± 70 | ||
| IGFBP3, pg/mL | Less than 5 years | 17218 ± 7742 | 0.23 |
| Older than 5 years | 19798 ± 6076 |
We didn’t find a statistically significant difference in pulmonary or pancreatic insufficiency between patients with and without growth failure (Table 3).
| Indices | Growth Failure | No. (%) | P Value |
|---|---|---|---|
| With pancreatic insufficiency | Yes | 8 (50) | 0.662 |
| No | 5 (41.7) | ||
| Without pancreatic insufficiency | Yes | 8 (50) | 0.229 |
| No | 7 (58.3) | ||
| With pulmonary involvement | Yes | 7 (43.8) | |
| No | 8 (66.7) | ||
| Without pulmonary involvement | Yes | 9 (5602) | |
| No | 4 (33.3) |
4.5. Correlation of Growth Peptides with Other Factors
A moderate association was found between age and level of IGF1 by using the Pearson test (r = 0.426, P = 0.004). No correlation was found between GH level and IGFBP3 with Pearson’s test. Also, there was no correlation between body mass index, height, and weight with growth peptides by using the Pearson test (P > 0.05).
No correlation was seen between GH and IGF1, (r = -0.247, P = 0.111), and IGFBP3 (r = -0.164, P = 0.294); also, no correlation was found between IGF1 and IGFBP3 (r = 0.257, P = 0.097) among children with cystic fibrosis by using the Pearson test.
5. Discussion
Surveys on cystic fibrosis in Iran are very limited. In a study on a population of CF children younger than 15 years old with respiratory symptoms, which was conducted in Iran, Modaresi et al. showed that 17.6% of all participants were positive for sweat test and only 1% of children required mutation assessment of cystic fibrosis transmembrane conductivity regulator gene to help confirm the diagnosis (23). CF is caused by mutations in the gene that encodes protein CFTR5 (24, 25). It is characterized by exocrine glands, including pancreas as the most important. Clinical features of Cystic Fibrosis include obstructive disorders in different ducts, and pancreatic insufficiency is the most common complication (26). Cystic Fibrosis is considered as the most common cause of disorders in the exocrine part of the pancreas in children. Nearly 85% of patients with CF show pancreatic insufficiency. Malabsorption due to pancreatic malignancy is the major clinical problem in patients, who survive after the period of infancy (27, 28). Rapid diagnosis and treatment of pancreatic insufficiency is an important issue in improving the health and outcome of CF patients. In the current study, steatorrhea was the most common symptom (46%), which is clearly an obvious sign of pancreatic insufficiency. In the current study, the mean age of children at the onset of CF was 3.4 ± 1.1 months and at diagnosis, this was 11.84 ± 3.09 months, while in Fallahi et al., found the mean age of children three and five months, respectively (29). It seems that the diagnosis occurrs too late and figuring out interference factors leading to delay is needed.
In the current study, the first three symptoms in children were steatorrhea (46%), cough (39.3%), and pneumonia (39.35%), while in Fallahi et al.’s study the growth failure was prominent (45%) (29), and less commonly, gastrointestinal and respiratory symptoms were also reported. The current findings showed the same percentage of growth failure in the studied children. The study included fewer CF patients and was of short-term duration, while study conducted by Fallahi et al. lasted 10 years longer. Various studies have shown that frequent airway infections and GI disorders, such as malabsorption and associated complications of pancreatic insufficiency, are major problems among CF patients (30-32). In many cases, it is also responsible for many cases of polyposis, rectal prolapse, pancreatitis, biliary stones, insulin-dependent hyperglycemia, growth failure, and liver function disorder. Bessich et al. showed that a low level of IGF1 in alveolar macrophages causes impairment in its performance among CF patients (33). This issue in explanation of the increasing risk of respiratory infections can be beneficial.
Ahasic et al. showed that there was a relationship between mean levels of IGF1, IGFBP3, and age (34); inconsistently, the current findings did not show a correlation between GH, IGFBP3, and age.
Dooghe et al. showed that there was a significant association between level of insulin and IGFBP3 with severity of growth failure (35), which is in alignment with the current findings. Taylor et al. reported a close correlation between the low level of serum IGF1, IGFBP3, and low BMI in patients with cystic fibrosis (36), while in the current study, no correlation was observed between mean serum levels of GH, IGF1, and IGFBP3 with growth indices, such as weight, height, and BMI among CF children, the mean serum levels of GH, IGF1, and IGFBP3 in CF children with growth failure were lower than children without growth failure; yet this difference was only significant for IGFBP3.
Street et al. showed that inflammation as a major modulator in IGF/IGFBP system causes reduction of IGF bioavailability in patients with CF (37). The current study showed that the mean serum levels of IGF1 and IGFBP3 in patients with pancreatic insufficiency and pulmonary involvement were lower than the patients without these complications, however, these differences were not statistically significant.
In the current study, 45% of children showed growth failure, while Gordon et al. showed that IGF1 has a strong association with bone mass index in CF patients (38). Considering the age of patients in these two studies (children in our study and adult in Gordon’s), this finding showed the importance of GH and related peptides in increasing height in pediatric CF patients.
5.1. Conclusions
The mean serum levels of GH, IGF1, and IGFBP3 in CF children with growth failure were lower than those in children without growth failure, yet no association was observed between pancreatic and pulmonary involvement with growth hormone levels and related peptides. Regarding the relatively high prevalence of growth disorders in children with cystic fibrosis, selected, appropriate, and standard medical and nutritional interventions can decrease the complications and burden of this chronic disease.
5.2. Strength of the Study
The aim of this study was to assess the level of growth-related hormones in CF patients. Basal GH level in serum does not have normal value and GH secretion is pulsatile so that the GH basal level may be similar in normal children and children with growth failure, thus, the researchers used IGF1 and IGFBP3 besides GH level, which did not have GH level limitation.