1. Background
The major clinical manifestation of meconium aspiration syndrome (MAS) is respiratory distress in a newborn due to the inhalation of amniotic fluid contaminated with meconium by the fetus while in the uterine cavity or during delivery. MAS occurs mainly in full-term or overdue infants and occasionally in preterm infants (1, 2). As a severe lung disease, MAS causes 10% - 20% of patients to develop pneumothorax, with a mortality rate of 5% - 10% (2-4). Therefore, studying effective treatment measures to improve the prognosis of MAS has long been an important topic that has attracted much attention from neonatal physicians. Based on full mastery of MAS ultrasound diagnostic technology (5, 6) and successful treatment of neonatal pulmonary atelectasis or massive pleural effusion using bronchoalveolar lavage (BAL) under point-of-care lung ultrasound (POC-LUS) monitoring (7, 8), the present study explored the treatment of MAS using BAL under ultrasound monitoring and obtained remarkable efficacy, thereby changing the traditional concept of MAS management. The results are reported as follows.
1.1. Subjects
This work was co-approved by the research ethics committee of Beijing Chaoyang District Maternal and Child Healthcare Hospital and the Army General Hospital of the Chinese PLA (No. E-LUS-08002). A total of 120 neonatal patients who were hospitalized in the neonatal intensive care unit (NICU) of our hospital between August 2015 and July 2018 and who were confirmed to have MAS according to their perinatal medical history (such as fetal distress, severe birth asphyxia, and meconium staining of amniotic fluid [MSAF]), clinical manifestations (respiratory distress shortly after birth), laboratory examinations and lung ultrasound (LUS) examinations were enrolled as study subjects. POC-LUS was performed immediately on admission. The diagnosis of MAS was based on the following critera (5, 6, 9): (1) Lung consolidation accompanied by air bronchograms with an irregular or jagged boundary was the most important sonogram characteristic of MAS. (2) The pleural line was abnormal, and the A-line disappeared. (3) The B-lines were visible or alveolar-interstitial syndrome (AIS) was apparent in the nonconsolidated areas. (4) Some patients had different degrees of unilateral or bilateral pleural effusion.
Patients were randomly divided into 2 groups according to their odd and even hospital numbers. The BL treatment group included 70 cases of 39 male and 31 female patients; their gestational ages (GAs) were between 30+6 and 42+2 weeks (among them, there were 8 infants with a GA of 30 - 35+6 weeks, 15 infants with a GA of 36 - 37 weeks, and 47 infants with a GA of > 37 weeks), and their body weights at birth were between 1350 and 4150 g. The control group included 50 cases of 27 male and 23 female patients; their GAs were between 31+3 and 41+4 weeks (among them, there were 5 infants with a GA of 31 - 35+6 weeks, 9 infants with a GA of 36 - 37 weeks, and 36 infants with a GA of > 37 weeks), and their body weights at birth were between 1300 and 4080 g.
2. Methods
2.1. Instruments and Examination Methods
Instruments included GE Voluson S9 and Logiq E10 (GE, USA) or Philips EPIQ 5 (Philips, The Netherlands) ultrasound diagnostic apparatuses and a linear array probe with a frequency of 10 - 14 MHz. Patients were placed in a prone, lateral, or supine position, and the probe was placed perpendicular or parallel to their ribs to scan all regions of the bilateral lungs (8).
2.2. Lavage Method
The method was based on previous experience with some modifications (7), with the specific procedure as follows: Based on their GAs and body weights at birth, MAS patients in the BAL treatment group received an injection of 1.5 - 3.0 mL of normal saline (0.9% sodium chloride injection) via a tracheal intubation apparatus. (1) For patients who received ventilator treatment, the ventilator parameters were appropriately increased before injection of the lavage fluid. Thus, based on the original parameters, the peak inspiratory pressure (PIP) was increased by 3 - 5 cm H2O, the positive end-expiratory pressure (PEEP) was increased by 2 - 3 cm H2O, Ti was prolonged to 0.55 - 0.60 s, the respiratory rate (RR) was increased by 10 - 20 times/min, and the fraction of inspired oxygen (FiO2) was increased based on the specific condition. After each injection of lavage fluid, ventilation was performed for 20 - 30 min. Then, sputum was suctioned through the tracheal intubation under negative pressure. BAL could be repeated 1 - 2 times based on the area of lung consolidation, which was considered to be 1 treatment course. Based on the condition of the patient, 2 - 3 treatment courses could be repeated each day. Afterwards, the need for another lavage treatment was determined based on the condition of pulmonary recruitment. (2) For infants who did not receive ventilator treatment or for whom the apparatus was already withdrawn, endotracheal intubation was performed again for endotracheal irrigation. In addition, based on the specific condition of the patient, a resuscitation bag was used for positive pressure ventilation, or a ventilator was connected for assisted aspiration. (3) POC-LUS was immediately performed after each course of lavage treatment. In addition, the need for another course of lavage treatment and the continuation of ventilator treatment were determined based on the ultrasonography results.
2.3. Observation Indicators
The observation indicators included the rate of invasive ventilator use, duration of ventilator use, incidence of persistent pulmonary hypertension of the newborn (PPHN) and/or pneumothorax, mortality, duration of hospitalization of the patients, and hospitalization expenses of patients in the 2 groups.
2.4. Statistical Methods
Data analysis was conducted using SPSS 18.0 for Windows. Differences in the duration of mechanical ventilation, duration of hospitalization, and hospitalization expenses are reported as the mean ± SD and were assessed by Student’s t-test. The significance levels of the differences in the rate of mechanical ventilation use, incidence rate of PPHN, and mortality between the 2 groups were assessed using χ2 tests and logistic regression analyses. A value of P < 0.05 was considered statistically significant.
3. Results
3.1. Comparison of Major Results Between the Two Groups of Patients
As shown in Table 1, BAL treatment under ultrasound monitoring significantly reduced the rate of mechanical ventilation use in MAS patients (P < 0.01); shortened the duration of ventilator use (P < 0.01); decreased the incidence of complications such as PPHN, pneumothorax, and lung bleeding (P < 0.01); decreased the mortality of severe patients, and reduced the hospitalization expenses (P < 0.01). None of the patients who received BL treatment had obvious side effects or treatment-related complications.
| Groups | IMV, No. (%) | DMV, h | Complicationsa | Hospital Costs, Yuan |
|---|---|---|---|---|
| BAL group (n = 70) | 19 (27.1) | 24.7 ± 5.3 | 3 (4.3) | 11545 ± 977 |
| Control group (n = 50) | 32 (64.0) | 166.2 ± 24.7 | 14 (28) | 20117 ± 1109 |
| Percent decrease, % | 57.7 | 85.1 | 84.6 | 42.6 |
| P value | < 0.01 | < 0.01 | < 0.01 | < 0.01 |
Comparison of Major Results from the Two Groups of Patients
Additionally, BL shortened the duration of hospitalization by 30% on average (P < 0.01), which was more significant in late-preterm infants and full-term newborns (Table 2).
| Groups | ≤ 35 Weeks | 36 - 37 Weeks | ≥ 37 Weeks | Average |
|---|---|---|---|---|
| BAL group | 13.7 ± 2.31 | 8.1 ± 1.93 | 6.1 ± 1.21 | 9.3 ± 1.82 |
| Control group, N | 17.8 ± 3.77 | 12.1 ± 2.11 | 9.9 ± 2.01 | 13.3 ± 2.71 |
| Percent decrease, % | 23.0 | 33.1 | 38.4 | 30.1 |
| P value | < 0.05 | < 0.01 | < 0.01 | < 0.01 |
Influence of BAL on the Length of the Patient’s Hospital Stay (Days)a
3.2. Description of Typical Cases
3.2.1. Case 1
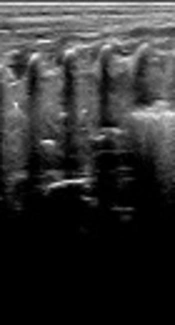
A female patient had a GA of 38+6 weeks, fetal distress, severe asphyxia at birth, MSAF, and a body weight of 3750 g at birth. The patient was admitted to the hospital due to severe dyspnea after resuscitation. POC-LUS (volume panorama mode) showed a large area of lung consolidation with air bronchograms and irregular margins in the whole right lung field (Figure 1A). Endotracheal intubation and BL treatment were performed immediately. After 2 rounds of BAL, LUS showed a nearly total normal lung appearance, with the complete disappearance of consolidation and only several B-lines (Figure 1B).
3.2.2. Case 2
A female patient had a GA of 41 weeks, intrauterine distress, severe asphyxia at birth, MSAF, and birth weight of 4000 g. The patient was admitted to the hospital 4 h after birth due to 4 h of dyspnea. POC-LUS showed a large area of lung consolidation combined with air bronchograms in the left lung (Figure 2A). Endotracheal intubation and BL treatment were performed immediately. After 1 treatment course (3 times of lavage), LUS showed that the lung consolidation completely disappeared, and the lungs returned to normal (Figure 2B).
4. Discussion
MAS is a common serious neonatal disease. The incidence of MSAF is associated with GA; an older GA results in a higher incidence of MSAF, with an overall incidence of approximately 12%. The incidence among infants with a GA of > 42 weeks can reach more than 30%. The incidence among infants with a GA of < 37 weeks is lower than 2%, and infants with a GA of < 34 weeks rarely develop MSAF (2, 10). Recently, however, we have found that the incidence rate of MAS in preterm infants was increased compared to that previously determined, including in newborns with a GA of < 32 weeks. The fetus is affected by adverse intrauterine factors before birth, inducing fetal respiratory movement, causing meconium particles to be inhaled into the distal airway. If the patient inhales a large amount of viscous meconium, severe dyspnea can occur shortly after birth, with significant or severe presentations such as tachypnea, nasal flaring, retraction, cyanosis, respiratory failure, PPHN, multiple organ failure, or even death. Rapid diagnosis and timely as well as proper treatment are the key points to improving the patient prognosis. The results of this study confirmed that LUS combined with BAL can achieve such results.
The results of this study showed that the efficacy of MAS treatment using BAL under ultrasound monitoring is remarkable. Because the disease is severe and its progression is rapid, the majority of patients require immediate mechanical ventilation, which has been a major method of MAS treatment for a long time. However, mechanical ventilation has significant side effects such as various complications (including ventilator-related complications). In addition, MAS is associated with a certain mortality rate. Therefore, the investigation and search for simple, effective, and new treatment methods to reduce complications and improve the prognosis to the greatest extent possible is one of the long-standing challenges faced by neonatal physicians. The results from this study indicate that treatment of neonatal MAS using BAL under LUS monitoring has outstanding advantages, such as decreasing the rate of mechanical ventilation use in MAS patients by 57.7%, shortening the duration of required mechanical ventilation by 85.1%, decreasing the incidence rate of MAS complications (such as PPHN, pneumothorax and lung bleeding, etc.) by 84.6%, reducing the mortality rate from 2% to 0%, shortening the duration of hospitalization by 30.1%, and reducing hospitalization expenses by 42.6%. Long-stay admissions have been reported to significantly increase a patient’s mortality (11), thus our results demonstrating that BAL can significantly shorten a patient’s hospital stay have important clinical significance. Furthermore, BAL is simple, causes no significant side effects or complications, and avoids radiation damage; thus, it is worthy of clinical application.
The BAL methods are as follows (7, 12): (1) The volume of lavage fluid used each time is based on the GA, birth weight, and disease course and is preferably 1.5 - 3.0 mL each time. An insufficient amount does not allow dilution of meconium-like substances, while an excessive amount may affect lung ventilation and air exchange, temporarily aggravating dyspnea. (2) According to the amount of patient sputum and the disappearance of lung consolidation after lavage, each treatment course may include 1 - 3 lavage repetitions, and a thorough, gentle backslap with higher frequency and sputum aspiration should be performed on the infants after each lavage. (3) Appropriate increases in ventilator parameter values: for patients receiving ventilator treatment, ventilator parameter values should be appropriately increased before the injection of lavage fluid. After each injection of lavage fluid, ventilation should be performed for more than 20 min to fully humidify consolidated lung tissues and dilute meconium-like substances so that they can be easily removed. (4) For patients not receiving ventilator treatment or if the ventilator was already withdrawn, endotracheal intubation should be performed, and a resuscitation bag should be used, or a ventilator should be connected for positive pressure ventilation. (5) After the completion of each lavage or each course of lavage treatment, lung ultrasonography should be immediately performed. The need for the next lavage or lavage treatment course should be decided based on ultrasonography.
Surfactant lavage therapy has been recommended for neonatal MAS with the benefits of a decreased neonatal death rate or a reduced need for extracorporeal membrane oxygenation (13); however, exogenous pulmonary surfactant is too expensive, especially for low-income families and those in developing countries. Our studies confirmed that normal saline can also achieve the same excellent efficiency, and saline is very inexpensive; thus, this method is more suitable for low-income families and for hospitals in developing countries.
4.1. Summary
In summary, this study investigated a new method of meconium aspiration syndrome treatment using BAL under ultrasound monitoring. The results showed that this method has remarkable efficacy, changing our traditional concepts of managing MAS patients, and did not exert any adverse side effects or complications. This method is simple, is easy to learn, can be performed at the bedside, and can facilitate the observation of a patient’s condition for timely treatment; thus, it deserves extensive clinical application. As with other technologies (14), appropriate training will aid in the effectiveness of BAL treatment in infants with MAS. The limitation of this paper is one should learn the LUS technique itself firstly before can make the BAL methods,to do this,one can follow the protocol and guidelines for neonatal LUS (15).