1. Background
Henoch-Schönlein purpura (HSP) is a systemic vasculitis characterized by the aggregation of immune complexes containing immunoglobulin A (IgA) into the walls of small vessels. It is also the most usual vasculitis in children, manifested by purpura, arthritis or arthralgia, abdominal pain and kidney disease. While purpura is a mandatory criterion for the diagnosis of HSP, other symptoms and signs are more diversely present (1).
HSP is a common form of pediatric vasculitis. The epidemiology of pediatric primary vasculitis varies in different regions and populations (2). The reported annual incidence is 6 to 30 cases per 100,000 children younger than 17 years for multi-ethnic regions (3-8). Gardner-Medwin reported the annual incidence in the United Kingdom as 20.4 cases per 100,000 children. The Indian subcontinent was reported to have a greater incidence (24 cases per 100,000) compared with White Caucasians (17.8 cases per 100,000) and Blacks (mainly Afro-Caribbeans) 6.2 cases per 100,000 (3). The annual incidence in the Netherlands and the Czech Republic was 6.1 and 10.2 cases per 100,000 children respectively (4, 6). Yang reported that the annual incidence was 12.9 cases per 100,000 children in Taiwan (7). The annual incidence in China was 14.06 cases per 100,000 children and the ratio of male to female was 1.30:1 to 1.41:1 in different parts of China (8, 9). Rostoker reported a male predominance (gender ratio of 1.5:1) with HSP (10), but recent studies suggest that there is equal incidence among both males and females (11). HSP usually affects children aged 3 to 10 (10, 12) with an average age of 6 (13). The factors causing HSP include potential infection, anesthesia, insect bites, toxins, food, season, drugs and vaccination. However, most HSP cases occur before upper respiratory tract infection (14, 15). The epidemiology of pediatric primary vasculitis in Northwestern China is still unclear. The aim of this study was to describe the epidemiology and clinical characteristics of HSP in this region.
HSP has a fine prognosis in most affected children. However, a few children will suffer from severe gastrointestinal tract involvement and kidney damage. Therefore, to predict this risk, we need better bio-markers that can reflect the phase and severity of HSP and hence support its clinical diagnosis.
2. Objectives
Our research sought to perform the following tasks: The epidemiological profile and clinical characteristics of children with HSP in Northwestern China were investigated for the first time; and then comprehensive laboratory inflammation and activated coagulation markers were evaluated, with the aim of identifying specific bio-markers in the progression of HSP. The combination of epidemiological profile, clinical characteristics and the identification of specific bio-markers could facilitate clinical diagnosis and determine the progression of HSP.
3. Methods
We retrospectively analyzed the clinical data of 135 patients diagnosed with HSP over a 2-year period (January 2016 to December 2017) at the Children's Hospital of Gansu province, China. The inclusion criteria for participation in this study were: (1) children aged ≤ 14 years, (2) children diagnosed with HSP on the basis of standard diagnostic criteria of EULAR/PRINTO/PRES for HSP (16). Patients with other diseases were excluded from the research. In the control group there were 86 randomly selected healthy children. Patients were divided into four groups according to the presence of (1) skin purpura, (2) acute arthritis or acute arthralgia, (3) gastrointestinal involvement (abdominal pain, occult blood in stool, melena, or hematochezia), (4) renal involvement (proteinuria or hematuria).
All clinical profile and blood test results were retrieved from patients’ medical records. Levels of C-reactive protein (CRP), procalcitonin, white blood cells count (WBC), neutrophils count, lymphocytes count, monocytes count, eosinophils count, hemoglobin; platelets count and mean platelet volume (MPV) were obtained from the complete blood count test results. Prothrombin time (PT), activated partial thromboplastin time (APTT), D-dimer and fibrin degradation product (FDP) levels were obtained from routine coagulation test results. The neutrophil-to-lymphocyte ratio (NLR), monocyte-to-lymphocyte ratio (MLR) and platelet-to-lymphocyte ratio (PLR) were calculated according to the results of whole blood cell count test.
To investigate the differences in clinical manifestation according to age, patients were categorized in two groups based on whether they were younger or older than 7 years. Continuous variables were represented by the mean of standard deviation (SD) or median (quartile spacing). Classified variables were represented by numbers and proportion. χ2 test or Fisher’s exact tests were used to perform comparisons between the two groups and the correlation between two variables was determined by Pearson’s correlation analysis. Multivariate logistic regression analysis was used to determine the odds ratios and 95% confidence intervals (CIs). To evaluate the predictive performance of biomarkers for disease course, one receiver operating characteristic curves (ROC) was plotted. SPSS statistical version 22.0 was used for all statistical analyses (IBM Corp., Armonk, NY, USA). P < 0.05 was considered to have statistical significance.
4. Results
4.1. Clinical Manifestation
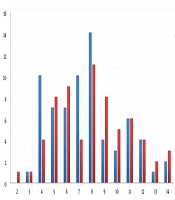
The main clinical characteristics of HSP patients are summarized in Table 1. Children with HSP participated in the analysis, comprised of 70 boys and 65 girls. The boy to girl ratio was 1.08:1. The average age of patients was 7.84 ± 2.81 (range 2 to 14) years, the relatively high incidence age of patients was 6 to 9 years (Figure 1). There were 135 (100%) patients with skin purpura, 77 (57.0%) with arthritis, 46 (34.1%) with gastrointestinal involvement, and 15 (11.1%) with renal involvement. The onset of HSP occurred throughout the seasons, but the peak season was winter (33.3%). Upper respiratory infection was the most common (40.7%) trigger for HSP. All patients were simultaneously tested for allergens. The total Immunoglobulin E (IgE) positive rate for allergens was 100%, while the highest positive rate for inhaled allergens were reported for elm/willow pollen (12.6%) followed by animal dander (8.2%).
| Characteristic | Value |
|---|---|
| Age, y | 7.84 ± 2.81 |
| Gender, male/female | 70/65 (1.08:1) |
| Clinical manifestations | |
| Purpura | 135 (100.0) |
| Arthritis | 77 (57.0) |
| Gastrointestinal involvement | 46 (34.1) |
| Renal involvement | 15 (11.1) |
| Seasons | |
| Spring | 35 (25.9) |
| Summer | 21 (15.6) |
| Autumn | 34 (25.2) |
| Winter | 45 (33.3) |
| Predisposing factors | |
| Upper respiratory infection | 55 (40.7) |
| Lower respiratory infection | 22 (16.3) |
| Other inflammatory conditions | 9 (6.7) |
| Allergens | 10 (7.4) |
| Unknown | 39 (28.9) |
| Total IgE positive rate for allergens | 135 (100.0) |
| Elm/willow pollen | 17 (12.6) |
| Animal dander | 11 (8.2) |
| Fruits | 4 (3.0) |
| Sagebrush/ragweed | 3 (2.2) |
| Dust acarid | 2 (1.5) |
| Shrimp | 2 (1.5) |
| Egg white | 1 (0.7) |
Distribution of the Clinical Features of Henoch-Schönlein Purpura Patients (N = 135)a
4.2. Differences in Clinical Manifestation According to Age
The differences in the clinical characteristics according to the different age groups (≤ 7 years or > 7 years) of patients are summarized in Table 2. As age increased, renal involvement syndrome became more frequent while the allergen positive rate decreased among HSP patients older than 7 years (P < 0.05). No differences were found regarding gender and other clinical symptoms between the two groups.
| Variable | Younger Age (≤ 7 y), N = 62 | Older Age (> 7 y), N = 73 | P Value |
|---|---|---|---|
| Age, y | 5.37 ± 1.23 | 9.95 ± 1.93 | - |
| Gender, male/female | 35/27 (1.30:1) | 35/38 (0.92:1) | 0.388 |
| Purpura | 62 (100.0) | 73 (100.0) | 1.000 |
| Arthritis | 40 (64.50) | 37 (50.70) | 0.194 |
| Gastrointestinal involvement | 17 (27.40) | 29 (39.70) | 0.112 |
| Renal involvement | 4 (6.40) | 11 (15.10) | 0.050 |
| Allergen-specific IgE | 21 (61.80) | 13 (38.20) | 0.016 |
Comparison of Patients by Age Groupa
4.3. Differences in Laboratory Bio-Markers Between Patients and Controls
The results of laboratory bio-markers in patients and controls are summarized in Table 3. Overall, 86 healthy children were enrolled in this study as controls, comprised of 46 male and 40 female children. The boy to girl ratio was 1.15:1. The average age was 7.01 ± 3.57 (range 2 to 14) years. In this study, there was no significant difference in sex and age between HSP patients and control group. The average WBC count, neutrophils count, platelets count, D-dimer, FDP levels, NLR and PLR were remarkably higher among HSP patients compared with the control group (P < 0.001).
| Variable | HSP Group, N = 135 | Control Group, N = 86 | P Value |
|---|---|---|---|
| Age, y | 7.84 ± 2.81 | 7.01 ± 3.57 | 0.070 |
| Gender, male/female | 70/65 (1.08:1) | 46/40 (1.15:1) | 0.890 |
| C-reactive protein, mg/L | 6.09 ± 10.3 | 2.26 ± 1.77 | < 0.001 |
| Procalcitonin, ng/mL | 0.079 ± 0.20 | - | - |
| Red blood cell, × 109/µL | 4.57 ± 0.49 | 4.61± 0.37 | 0.550 |
| Hemoglobin, g/L | 130.5 ± 13.2 | 126.8 ± 13.1 | 0.040 |
| White blood cell, × 106/µL | 8.85 ± 3.48 | 7.29 ± 2.21 | < 0.001 |
| Lymphocyte count | 3.26 ± 2.21 | 2.98 ± 1.14 | 0.268 |
| Monocytes count | 0.57 ± 0.28 | 0.51 ± 0.24 | 0.102 |
| Eosinophil count | 0.11 ± 0.15 | 0.09 ± 0.07 | 0.070 |
| Platelet, 1 × 106/µL | 297.9 ± 106.9 | 238.9 ± 53.9 | < 0.001 |
| MPV, fl | 10.58 ± 1.43 | 10.13 ± 1.26 | 0.020 |
| NLR | 2.09 ± 2.27 | 1.33 ± 0.66 | 0.003 |
| APTT | 33.75 ± 6.43 | 35.43 ± 5.60 | 0.048 |
| D-dimer | 1.51 ± 2.14 | 0.55 ± 0.42 | < 0.001 |
| FDP | 5.79 ± 7.69 | 2.92 ± 1.58 | < 0.001 |
Comparison Between Characteristics of Henoch-Schönlein Purpura Patients and Controlsa
4.4. Differences in Laboratory Bio-Markers Between Groups, with or Without Gastrointestinal Involvement
The results of laboratory bio-markers among HSP patients with or without gastrointestinal involvement are summarized in Table 4. The WBC count, neutrophil count, monocyte count, hemoglobin, APTT, D-dimer and FDP levels differed significantly between the two groups (P < 0.05).
| Variable | GI (-), N = 77 | GI (+), N = 43 | P Value |
|---|---|---|---|
| Age, y | 7.55 ± 0.98 | 8.06 ± 2.69 | 0.799 |
| Gender, male/female | 44/33 (1.33:1) | 19/24 (0.79:1) | 0.187 |
| C-reactive protein, mg/L | 5.40 ± 8.77 | 7.47 ± 14.33 | 0.408 |
| Procalcitonin, ng/mL | 0.08 ± 0.19 | 0.09 ± 0.27 | 0.891 |
| Red blood cell, × 109/µL | 4.65 ± 0.38 | 4.63 ± 0.61 | 0.861 |
| Hemoglobin, g/L | 133.78 ± 9.98 | 131.9 ± 15.6 | 0.498 |
| White blood cell, × 106/µL | 7.50 ± 2.52 | 9.79 ± 4.11 | 0.002 |
| Lymphocyte count | 3.26 ± 3.18 | 3.19 ± 1.52 | 0.901 |
| Monocyte count | 0.45 ± 0.20 | 0.63 ± 0.36 | 0.009 |
| Eosinophil count | 0.12 ± 0.09 | 0.09 ± 0.17 | 0.344 |
| Platelet, × 106/µL | 278.47 ± 86.43 | 296.6 ± 120 | 0.413 |
| MPV, fl | 10.64 ± 1.48 | 10.72 ± 1.42 | 0.805 |
| NLR | 1.89 ± 2.66 | 2.41 ± 2.12 | 0.317 |
| MLR | 0.19 ± 0.18 | 0.22 ± 0.13 | 0.392 |
| PLR | 106.82 ± 44.30 | 115.1 ± 65.9 | 0.495 |
| PT | 12.41 ± 0.92 | 12.37 ± 1.26 | 0.891 |
| APTT | 35.81 ± 6.27 | 32.19 ± 6.99 | 0.066 |
| D-dimer | 0.78 ± 1.23 | 1.73 ± 2.26 | 0.019 |
| FDP | 3.31 ± 3.37 | 6.62 ± 7.52 | 0.011 |
Comparison Between Groups with or Without Gastrointestinal Involvementa
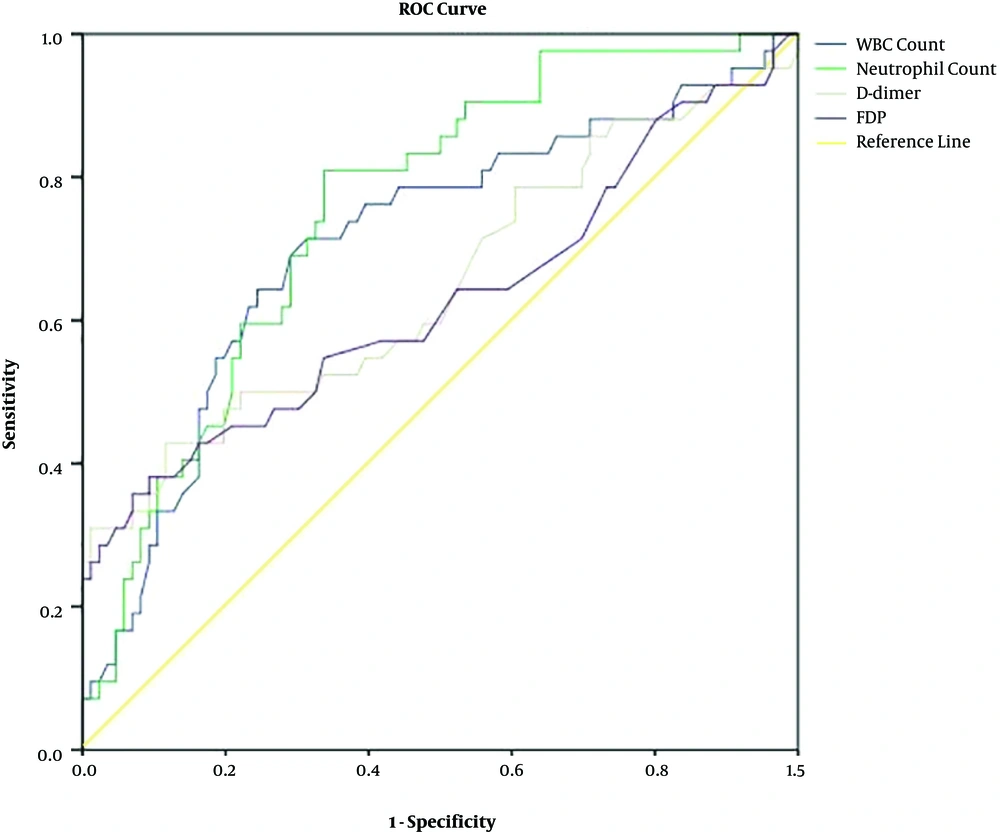
ROC curves of the four markers in patients with gastrointestinal involvement are presented in Figure 2. The areas under the curve for WBC count (0.7, 95% CI, 0.61 - 0.81), neutrophil count (0.76, 95% CI, 0.67 - 0.84) and D-dimer (0.65, 95% CI, 0.54 - 0.76) are shown below. The lowest area under the curve (0.63, 95% CI, 0.51 - 0.74) occurred with FDP.
4.5. Differences in Laboratory Bio-Markers Between Groups, with or Without Renal Involvement
The results of laboratory bio-markers of patients with or without renal involvement are summarized in Table 5. D-dimer and hemoglobin levels were significant differences between two groups. The mean age for the Henöch-Schonlein purpura nephritis (HSPN) group was slightly higher than that for the non-HSPN group.
| Variable | HSPN (-), N = 120 | HSPN (+), N = 15 | P Value |
|---|---|---|---|
| Age, y | 7.74 ± 2.87 | 8.67 ± 2.22 | 0.233 |
| Gender, male/female | 63/57 (1.10:1) | 7/8 (0.875:1) | 0.786 |
| C-reactive protein, mg/L | 6.38 ± 11.73 | 5.51 ± 6.62 | 0.645 |
| Procalcitonin, ng/mL | 0.08 ± 0.29 | 0.08 ± 0.03 | 0.905 |
| Red blood cell, × 109/µL | 4.64 ± 0.50 | 4.43 ± 0.46 | 0.115 |
| Hemoglobin, g/L | 132.88 ± 12.96 | 125.8 ± 12.4 | 0.003 |
| White blood cell, × 106/µL | 8.59 ± 3.54 | 9.34 ± 3.33 | 0.243 |
| Neutrophil count | 4.94 ± 3.26 | 5.23 ± 2.85 | 0.601 |
| Lymphocyte count | 3.23 ± 2.51 | 3.33 ± 1.46 | 0.797 |
| Monocytes count | 0.54 ± 0.30 | 0.62 ± 0.22 | 0.116 |
| Eosinophil count | 0.11 ± 0.13 | 0.12 ± 0.16 | 0.727 |
| Platelet, × 106/µL | 287.24 ± 103.87 | 318.9 ± 111.0 | 0.106 |
| MPV, fl | 10.68 ± 1.45 | 10.37 ± 1.37 | 0.236 |
| NLR | 2.14 ± 2.41 | 1.99 ± 1.97 | 0.721 |
| MLR | 0.20 ± 0.15 | 0.22 ± 0.13 | 0.554 |
| PLR | 110.80 ± 55.66 | 111.8 ± 61.3 | 0.921 |
| PT | 12.39 ± 1.09 | 12.41 ± 1.13 | 0.804 |
| APTT | 34.56 ± 6.72 | 32.04 ± 5.46 | 0.203 |
| D-dimer | 1.23 ± 1.85 | 2.12 ± 2.57 | 0.048 |
| FDP | 4.89 ± 5.94 | 7.61 ± 10.24 | 0.112 |
Comparison of Laboratory Bio-Marker Levels Between Groups, with or Without Renal Involvementa
5. Discussion
Henoch-Schönlein purpura is a systemic vasculitis characterized by the accumulation of immune complexes contained immunoglobulin A (IgA) on the walls of small vessels. It is also the most common vasculitis in children manifested by purpura, arthritis or arthralgia, abdominal pain and kidney disease. The epidemiology of pediatric primary vasculitis varies among regions and populations (2). Some studies have reported annual incidences ranging from 6 - 30 cases per 100,000 children aged < 17 years in multi-ethnic regions (3-8). This study included 135 children with HSP and 86 healthy controls with mean ages of 7.84 ± 2.81 years and 7.01 ± 3.57 years, respectively. Among the affected children, 52% and 48% were males and females, respectively, with a sex ratio of 1.08:1. This implies that HSP occurred more commonly among males, which is consistent with the findings of previous studies (8-10). However, a recent study suggested that there is equal incidence in both genders (11). This may be caused by regional and population differences.
According to our study, HSP could be observed during any season. However, the peak incidence occurred in the winter season (33.3%), while the lowest occurred during the summer season (15.6%), which is consistent with the findings of other studies (17, 18). The majority of HSP cases are preceded by infections (14, 15). Our study showed that upper respiratory tract infection was the major triggering factor in 40.7% of cases. This might be due to the poor quality of air and the significant change in temperature during winter which may easily precipitate respiratory tract infections in children (14). In this study, the incidence of skin purpura, arthritis, gastrointestinal and renal involvement among patients was 100%, 57.0%, 34.1% and 11.1%, respectively. The incidence of gastrointestinal and renal involvement was relatively lower compared to other Chinese studies (14, 19), which might be due to regional factors. The allergens for patients were also tested. The rates of sensitization to inhaled allergens were higher compared to those of ingested allergens, suggesting that respiratory allergic diseases are more closely related to HSP in Gansu province. The results of our study showed that the most inhaled allergens were elm/willow pollen and animal dander with positive rates of 12.6% and 8.2%, respectively. This might have arisen from the presence of more sensitization opportunities due to increasing levels of allergic substances in the air. Our results are different from those of other studies (20, 21) with the main allergen being dust mite, because most previous studies were conducted in coastal areas where the climate is humid and dust mites and cockroaches survive and spread more easily, while the drought climate in Northwest China is not conducive to the survival and spread of dust mites. We suggest that limiting allergen exposure in sensitive children can prevent allergic diseases. Some studies have shown that age (> 7 years) was a risk factor for renal involvement (17) and age at onset was a poor prognostic factor in HSPN patients (22). In this research, we divided the patients into two groups based on the cut-off age of 7-years. We found that the frequency of renal involvement was significantly higher among patients older than 7 years but lower among patients with allergen positivity, which was consistent with the report of Zhao et al. (17). This finding confirmed that the probability of renal involvement increases with age in HSP children.
Abdominal pain, vomiting and gastrointestinal bleeding are common symptoms in HSP patients. Gastrointestinal (GI) manifestation can be found in two thirds of cases (23). Alizadeh et al. (23) found GI symptoms in 77% of patients whereas a range of 34% to 75% was reported in other studies (24-26). However, some reports indicate that gastrointestinal bleeding is the most common symptom (24), while in other reports pain is the most common symptom (25). In our study, 34% of patients had gastrointestinal symptoms and abdominal pain was the most common symptom.
With the progress of immunology, biochemistry and molecular biology, there is new understanding about the development of HSP, including inflammatory, immunological, and coagulation mechanisms. Inflammation and coagulation systems are considered very important in the etiopathogenesis of vascular diseases. Inflammation activates coagulation which also severely affects inflammatory activity (27). No single unique laboratory test can be used to diagnose HSP. Several studies have reported on the relationship between laboratory markers and HSP (19, 28). However, the association between these markers with disease phases and severity are not fully understood. Further comprehensive studies are needed to investigate the role of laboratory markers in HSP phases and severity. Some studies have shown that platelets, leukocytosis and elevated CRP are associated with the severity of HSP, particularly with gastrointestinal bleeding in HSP (29, 30). Meanwhile, some scholars have also reported that lower mean platelet volume (MPV) may be associated with gastrointestinal bleeding in HSP (31). In addition, NLR and PLR, as serum markers reflecting inflammatory response, are widely used in systemic inflammatory diseases because they are cheap and can be easily and quickly utilized. They are also potentially useful indicators of inflammatory diseases indicating the stage and grade of the disease. Makay et al. (32) found that the NLR of pediatric HSP patients with gastrointestinal bleeding was significantly higher than that of patients without gastrointestinal bleeding. They reported that MPV and NLR were two significant factors for gastrointestinal bleeding in logistic regression analysis (32). In the study conducted by Park et al. (33), it was reported that adults with gastrointestinal bleeding had a higher NLR than those without gastrointestinal bleeding in HSP. In this study we obtained similar results, white blood cell count, NLR, PLR and platelets were significantly increased in children with HSP. These results are consistent with those of previous studies (19, 28). NLR does not fully reflect the complexity of the inflammatory response and immune response in Henoch-Schönlein purpura. However, Henoch-Schönlein purpura is an inflammatory disease with predominant neutrophil response, and it is reasonable to assume that high levels of NLR are associated with the immune response in Henoch-Schönlein purpura. While increased neutrophils and lymphopenia are usually observed in cases of infection, increased neutrophils and lymphopenia are also observed in cases of inflammation. Our study found no change in lymphocyte numbers despite increased neutrophil numbers and NLR in children with HSP. In this regard, our study is consistent with the study conducted by Makay et al. (32).
The body produces allergic reactions and synthetic antigen-antibody complexes damage the capillary endothelium causing vascular inflammation and leading to capillary fragility and increased permeability. Damaged vascular endothelial cells exposes the collagen fibers of vascular endothelium which activate the endogenous coagulation system and trigger the coagulation reaction. At the same time, prothrombin activation and fibrin deposition can stimulate the extrinsic coagulation system and render the patient's blood hypercoagulable. D-Dimer is a stable and specific degradation product of cross-linked fibrin, and its increased level indicates enhanced secondary fibrinolytic activity, which can be used as one of the molecular markers of hypercoagulability and hyperfibrinolysis in vivo (34). In particular, D-dimer has been found to be well correlated with the disease activity of HSP (35). FDP is the total degradation product of fibrin, fibrin monomer and cross-linked fibrin. It has more components, so its specificity is relatively lower than D-Dimer. In this study we also found that patients with gastrointestinal involvement had significantly higher D-dimer and FDP levels than those without gastrointestinal involvement (P < 0.05). Compared with the inflammatory markers such as the WBC count, NLR, PLR and CRP levels, D-dimer and FDP levels were more consistently elevated in the patients with gastrointestinal involvement. These results suggest that D-dimer and FDPs may play important roles as indicators of gastrointestinal tract involvement during the acute phase of HSP. Therefore, the fibrin markers D-dimer and FDP, enable clinical evaluation of not only the effectiveness of treatment but also disease recurrence, especially in young children complaining of recurrent abdominal pain during HSP treatment, which is consistent with the study conducted by Hong and Yang (28).
With organ damage in HSP, HSPN is a common secondary glomerulonephritis in pediatrics. The degree of renal damage is an important factor in determining the long-term prognosis of HSP. 20% to 54% of children with HSP may develop renal involvement (36, 37). However, 11% of children in our study had renal involvement which is relatively low. Although the pathogenesis of HSPN has not been thoroughly investigated, previous studies have implicated IgA-containing immune complex deposition in glomerular basement membrane (38). There are limitations to the identification of HSPN in the clinic setting and there is an urgent need to be able to identify bio-markers that will promptly reflect the disorders of renal function. Yilmaz et al. (35) reported that the level of D-dimer in HSP patients with renal impairment was significantly higher than that in patients without renal impairment. Other studies have reported similar findings (14, 39). The results of this study showed that comparing with other inflammatory markers, the D-dimer levels in HSP group and HSPN group was significantly higher than that in normal control group (P < 0.01), and the expression of D-dimer in HSPN group was higher than that in HSP group (P < 0.05), suggesting that D-dimer is correlated more strongly with the degree of renal injury and reflects the HSP phases and severity. Early clinical measurement of D-dimer level may not only detect HSP but can also serve as a signal for early renal damage. Early intervention and treatment can reduce the degree of glomerular and renal tubular interstitial damage in order to achieve the goal of slowing down glomerular disease progression.
5.1. Conclusions
To summarize, this is the first comprehensive study analyzing the epidemiology, allergen and laboratory biomarkers of both inflammation and activated coagulation and demonstrating their relationships with disease phases and severity in HSP using a clinical scoring system. Most HSP patients have joint involvement and the most common symptoms occurred in 6 - 9-year school-aged children during winter seasons. Upper respiratory infection is the most common trigger for HSP. Compared with the inflammatory markers, elevated D-dimer level is independently associated with gastrointestinal symptoms and renal involvement. However, the limitation of this research was that it was a single central and retrospective study. Despite the limitation, our data provided a better understanding of the process of HSP in children.