1. Background
Lead is a neurotoxicant with recognized health hazards to all age groups especially to developing children. It affects their cognitive, behavioral, and verbal development and intelligence quotient (IQ). Moreover, it has serious implications for their future performance and achievement. There is no safe lead level and even low levels might be harmful (1, 2).
According to the Egyptian Environmental Monitoring Center, the annual average of Total Suspended Particles (TSP) Concentration in Great Cairo in 2016 was estimated to be 421 µg/m3. That exceeds four times the maximum allowed level (125 µg/m3). Egyptians are exposed to high levels of lead every day. Lead-based paints (LBP), lead-contaminated dust and soil are the primary sources of lead exposure (3, 4).
Preterm infants are at increased risk for anemia of prematurity (AOP). AOP results from reduced gestational age and accordingly underdevelopment of the hematopoietic system. Packed red blood cells (PRBCs) transfusions are often used to manage AOP by increasing oxygen delivery to tissues. Thereby, it is a rapid and effective intervention to manage anemia. Thus, PRBCs transfusions may potentially increase blood lead level (BLL) delivered to preterm infants from the donor PRBCs aliquots (5).
A large proportion of PRBCs transfusions are given during the early weeks of life, a time when the excretory ability through urine and stool is most limited. Many adverse effects have been associated with receiving PRBCs transfusions such as necrotizing enterocolitis (NEC), intraventricular hemorrhage (IVH), retinopathy of prematurity (ROP) and bronchopulmonary dysplasia (BPD) (6, 7). In addition, pre-existing neonatal critical illnesses and the difficulties in defining adverse transfusion events in neonatal intensive care units (NICU), resulted in under-recognition and under-reporting of the transfusion-related adverse events (8).
2. Objectives
In this study, we hypothesized that premature infants receiving PRBCs transfusion may be exposed to a high lead level which could lead to increased post-transfusion BLL. The aim of this study was to evaluate the direct effects of PRBCs transfusions on neonatal blood lead levels among neonates admitted to Kasr El Ainy NICU of Cairo University Pediatric Hospital (CUPH).
3. Methods
This is a prospective cohort study of premature neonates. Preterm births (delivery before 37 completed gestational age); receiving at least one PRBCs transfusion were included in the study. They were admitted to Kasr El Ainy NICU at CUPH. Neonates with known congenital anomalies, received exchange transfusion, large-volume transfusions and transfusions used in surgeries were excluded.
The study took place over a period of 6 months starting from January 2018. From a total of 200 preterm neonates, fifty-four ones fulfilling the inclusion criteria were prospectively enrolled in the study.
3.1. Collected Data Included the Following
3.1.1. Neonatal Data
Age, sex, weight, gestational age, type of delivery, types of nutrition, primary diagnosis on admission.
3.1.2. Clinical Findings
(i) Complications during NICU stay (9).
(ii) Mortality: either mortality associated with receipt of PRBCs transfusion (within 24 - 48 hours of receipt of a transfusion), which may be related to initial pathology or mortality before discharge from initial diagnosis or complications.
3.1.3. Transfusion Details: Volume and Number of Transfusions
All the neonates received low volume 10 - 15 mL/kg in every time of transfusion. Adverse transfusion events within 48 hours of receipt of PRBCs such as:
(i) Immune-mediated transfusion reactions: as febrile transfusion reactions.
(ii) Acute non-immune-mediated transfusion reactions: as transfusion-related circulatory overload or metabolic complications.
3.1.4. Laboratory Findings
3.1.4.1. Creatinine Levels
To adjust for the expected improvement in glomerular filtration rate.
3.1.4.2. Blood Lead Levels (BLL)
For each neonate enrolled in the study, a baseline and post-transfusion BLL estimation was done.
A sample was collected at the first lab draw in NICU for the baseline while the post-sampling was timed six hours after the transfusion to allow time for equilibration of the transfused PRBCs, based on previous studies since there is no data currently available describing the optimal time for equilibration (1).
Neonatal BLL percent change was calculated to quantify the change levels before and after transfusion through the Equation 1:
Also, lead level from the transfused donor aliquot of PRBCs was obtained.
Lead testing was sent to the central lab of the ministry of health to determine lead amounts in the blood samples. The "PinAAcle900T" atomic absorption spectrometer (i.e. using the stabilized temperature platform furnace (STPF) and transversely-heated graphite atomizer (THGA) (Perkin Elmer Int., USA) was utilized. BLLs were reported with units of µg/dL.
The Joint Food and Agriculture Organization/World Health Organization Expert Committee on Food Additives has decided that there is no set intake safe level for lead. However, the IV reference dose would be 0.19 µg/kg/day (10).
Collected data were entered and analyzed using the Statistical Package for Social Science Software (SPSS) program, version 21.0 IBM. Tests of normality of data (like Shapero-Wilk test) revealed that data isn’t normally distributed. That’s why non-parametric tests like Mann-Whitney and Kruskal-Wallis tests were used in univariable comparisons to quantify the associations of continuous variables. Data were summarized using the median and interquartile range for quantitative variables. Box and whisker plots were used to show the evolution of BLL pre and post transfusion and to show BLL after transfusion in comparison with lead levels in PRBC packets. Spearman correlation test was used to detect relation between continuous variables. P values below 0.05 were considered statistically significant. Multivariate analysis using linear regression model was done to explore how well a set of continuous, neonatal variables (neonatal age, neonatal weight, gestational age, number of transfusion times, lead level in blood packs and blood creatinine level) are able to predict a particular outcome i.e. BLL% change and which variable in this set of variables is the best predictor of the outcome, when the effects of other variables are controlled for.
4. Results
The average age of neonates at transfusion was 12 days. Extreme low birth weight (< 1000 g) represented 5.6% of the sample. The median gestational age of the studied neonates was 33 weeks (IQR: 32 - 35) (Table 1).
| Variable | Descriptive (Total = 54 Neonates) | |
|---|---|---|
| Median (IQR) | Range | |
| Age at transfusion, days | 12 (7 - 18) | 1 - 50 |
| Gestational age, wk | 33 (32 - 35) | 26 - 35 |
| Neonates’ weight, g | 1720 (1360 - 1900 ) | 750 - 2300 |
| Laboratory characteristicsa | ||
| Neonatal BLL before transfusion | 1.5 (1.2 - 2.1) | 0.4 - 4.8 |
| Neonatal BLL after transfusion | 2.6 (2.3 - 4.2) | 0.5 - 9.9 |
| Neonatal BLL percent changeb | 72 (48.4 - 101.6) | 15.1 - 212.7 |
| Packed RBCs packets lead level | 3.9 (2.9 - 6.1) | 2.2 - 10.5 |
| Some transfusion characteristics | No. (%) | |
| Volume of transfusion | ||
| Low volume (10 - 15 mL/kg) | 54 (100) | |
| Number of transfusion times | ||
| Single | 34 (63) | |
| Twice | 8 (14.8) | |
| Three times | 9 (16.7) | |
| Four and more times | 3 (5.6) | |
| Neonatal weight groups, g | ||
| ELBW, < 1000 | 3 (5.6) | |
| VLBW, 1000 - 1500 | 12 (22.2) | |
| LBW, 1500 - 2500 | 39 (72.2) | |
| Mortality within 24 - 48 h | 6 (11.1) | |
Abbreviations: BLL, blood lead level; ELBW, extreme low birth weight infants; IQR, inter quartile range; LBW, low birth weight infants; RBCs, packed red blood cells; VLBW, very low birth weight infants
aBLL measured in (µg/dL).
bNeonatal BLL% change = [(BLL after - BLL before)/BLL before] ×100.
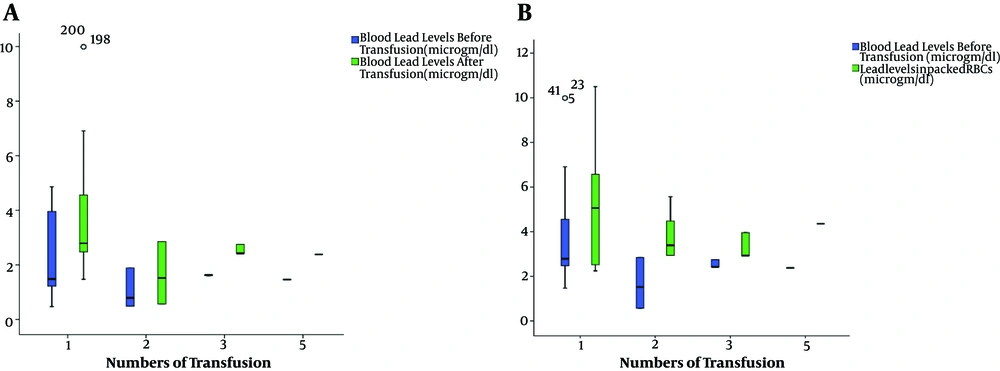
Box and whisker plots showed the evolution of BLL levels pre and post transfusion among neonates grouped according to the times of receiving transfusions. The median neonatal BLL after transfusion was elevated one and half times more than before transfusion (Figure 1A). Also, Box and whisker plots were used to show BLL after transfusion in comparison with lead levels in PRBC packets (Figure 1B). The lead level in the PRBCs packets ranged from 2.2 µg/dL to 10.5 µg/dL with median level 3.9 µg/dL (Table 1).
Meanwhile, the median neonatal BLL% change was significantly higher in cases of neonatal sepsis. Also, median BLL% change was significantly higher in neonates who died within 24 - 48 hours from transfusion (P < 0.001).
Neonates who suffered complications during NICU stay like BPD, ROP, IVH showed significantly lower median BLL% change. (P < 0.001) (Table 2).
| Variables | Neonatal BLL% Change, Median (IQR)a,b | P Value |
|---|---|---|
| Gender | 0.39 | |
| Males | 72.2 (63.9 - 92.4) | |
| Females | 50.7 (48.4 - 101.6) | |
| Type of delivery | 0.27 | |
| Vaginal delivery | 85.1 (63.9 - 101.6) | |
| Caesarian section | 63 (48.4 - 108.5) | |
| Type of feeding | 0.66 | |
| Total parental nutrition | 68 (48.4 - 101.6) | |
| Combined artificial formula breast feeding | 71.8 (50.7 - 132.8) | |
| Diagnosis on admissionc | 0.000 | |
| Neonatal pneumonia | 92.4 (15.1 - 108.5) | |
| Neonatal respiratory distress syndrome | 50.7 (48.4 - 71.8) | |
| PDA | 132.8 (132.8 - 133.2) | |
| Early onset neonatal sepsis | 168.2 (168.2 - 168.5) | |
| Neonatal convulsions | 63 (63 - 63.5) | |
| Transient tachyapnea of newborn | 85.1 (85.1 - 85.5) | |
| Complications during NICU stayb | ||
| Broncho pulmonary dysplasia | 0.001 | |
| Yes | 56.9 (48.4 - 72) | |
| No | 98.1 (63.9 - 132.8) | |
| Retinopathy of prematurity | 0.000 | |
| Yes | 49.6 (45.3 - 72.0) | |
| No | 98.1 (63.9 - 132.8) | |
| Late onset neonatal sepsis | 0.29 | |
| Yes | 71.8 (48.4 - 72.2) | |
| No | 85.1 (48.4 - 108.5) | |
| Intra ventricular hemorrhage | 0.000 | |
| Yes | 56.9 (42.1 - 72.2) | |
| No | 98.1 (74.5 - 120.7) | |
| Mortality within 24 - 48 h from transfusion | 0.001 | |
| Yes | 160.6 (108.5 - 212.7) | |
| No | 67.9 (48.4 - 93.5) |
Abbreviations: BLL, blood lead level; PDA, patent ductus arteriuosis.
aNeonatal BLL% change = [(BLL after - BLL before)/BLL before] × 100.
bP value was calculated using Mann-Whitney test.
cP value was calculated using Kruskal-Wallis test.
The median neonatal BLL% change was significantly higher in neonates who received one PRBCs transfusion 94.6 (63.9 - 132.8) rather than changes noticed among those who received two or more transfusions 63 (63 - 63) (P = 0.004) (Table 3).
| Variables | Neonatal BLL% Change, Median (IQR)a,b | P Value |
|---|---|---|
| Neonatal weightb | 0.16 | |
| ELBW | 101.6 (101.6 - 101.9) | |
| VLBW | 82.3 (49.6 - 182.6) | |
| LBW | 63.9 (48.4 - 94.6) | |
| Numbers of transfusionb | 0.004 | |
| Single | 94.6 (63.9 - 132.8) | |
| Twice | 50.7 (16.3 - 71.6) | |
| Three times | 48.4 (48.4 - 71.8) | |
| Four and more times | 63 (63 - 63.2) | |
| Complications related to transfusionc | ||
| Electrolyte imbalance | 0.12 | |
| Yes | 101.6 (101.6 - 101.9) | |
| No | 71.8 (48.4 - 94.6) | |
| Hypoglycemia | 0.39 | |
| Yes | 82.1 (60.1 - 130.3) | |
| No | 68 (48.4 - 101.6) | |
| Post-transfusion purpura | 0.21 | |
| Yes | 63.4 (48.47 - 94.6) | |
| No | 78.6 (49.63 - 120.7) | |
| Post transfusion sepsis | 0.000 | |
| Yes | 132.8 (108.5 - 168.2) | |
| No | 63 (48.4 - 72.2) |
Abbreviations: BLL, blood lead level; ELBW, extreme low birth weight infants; IQR, inter quartile range; LBW, low birth weight infants; VLBW, very low birth weight infants.
aNeonatal BLL% change = [(BLL after - BLL before)/BLL before] × 100.
bP value was calculated using Kruskal-Wallis test.
cP value was calculated using Mann-Whitney test.
Neonates who suffered post-transfusion sepsis showed significantly higher median BLL% change. (P < 0.001) (Table 3).
BLL after transfusion showed a positive, moderate and significant relationship with neonatal weight, lead level in blood packs, gestational age, and blood creatinine level respectively. Meanwhile, the neonatal BLL% change showed negative, significant correlation with neonatal age and number of transfusion times (Table 4).
| Variables | Blood Lead Levels After Transfusion, µg/dL, (r)a | P Value | Neonatal BLL% Changeb | P Value |
|---|---|---|---|---|
| Neonatal age, days | 0.020 | 0.888 | -0.385c | 0.004 |
| Gestational age, wk | 0.430c | 0.001 | 0.146 | 0.292 |
| Neonatal weight, g | 0.323* | 0.017 | 0.145 | 0.219 |
| Number of transfusion times | -0.390c | 0.004 | -0.474c | 0.000 |
| Lead level in blood packs | 0.360c | 0.008 | 0.252 | 0.066 |
| Blood creatinine level aftertransfusion | 0.514c | 0.000 | -0.17 | 0.219 |
| Linear Regression Model Showing Predictors of BLL% Changeb | ||||
| β= Beta Coefficient | CI | P Value | ||
| Neonatal age, days | -1.729 | -3.129 - -0.330 | 0.017 | |
| Gestational age, wk | -19.193 | -26.513 - -11.873 | 0.000 | |
| Neonatal weight, g | 0.118 | 0.072 - 0.164 | 0.000 | |
| Number of transfusion times | -16.074 | -26.242 - -5.905 | 0.003 | |
| Lead level in blood packs | 4.997 | 0.339 - 9.655 | 0.036 | |
| Blood creatinine level after transfusion | -33.642 | -53.861 - -13.423 | 0.002 | |
Abbreviations: BLL, blood lead level; CI, confidence interval.
a(r): Correlation coefficient, Spearman correlation test.
bR2 = 0.541.
cCorrelation is significant at the 0.01 level (2-tailed).
While comparing the contribution of each continuous neonatal variable in BLL% change, all of them showed a statistically significant contribution to the prediction of BLL% change. The largest Beta coefficient (33.6) was for blood creatinine level after transfusion, followed by gestational age (19.1) and number of transfusion times (16). This means that these three variables make the strongest, unique contribution to explaining the variance in BLL% change (Table 4).
5. Discussion
This study highlighted the direct effects of PRBCs transfusions on premature neonatal BLL. The median lead level (LL) in the PRBCs packets was remarkably higher than in similar studies. In a study conducted in Regional Medical Center, Memphis, the average LL in PRBCs packet was 1.9 ± 1.2 µg/dL, while in another study conducted in Massachusetts the average lead load per packet was 1.3 μg with a range of 0 - 8.6 µg (1, 6). The presence of low-dose lead toxicity in the population especially blood donors can be attributed to the increased level of lead use in Egypt (3). PRBCs are prepared from donors’ blood who are exposed to the polluted environment. There are no protocols to measure the BLL in donors’ blood similar to those used during screening for infectious diseases (11).
The current study revealed that the median neonatal BLL after transfusion was much elevated than that before transfusion. This goes in accordance with the study done by Zubairi et al. 2015, who showed that for each 1 µg/dL of transfused PRBCs, there was a 0.20 µg/dL increase in infant BLL (1). Multiple transfusions to premature infant can result in unacceptable values of post-transfusion lead levels. Potential sources of exposure to lead for premature infants may be either antenatal transmission or PRBCs transfusion (12).
The current study evinced that neonates who received their first PRBCs transfusion showed higher BLL than those received two or more transfusions. This indicates that neonates who received a single transfusion had lower BLL at baseline. Subsequently, the change after their first transfusion was significant. This can be justified by: First, the progressive improvement in glomerular filtration rate (GFR). Kidney function of low birth weight (LBW) infants in the 1st week of life is associated with increased lead re-absorption (6). Also, preterm neonates metabolize lead differently and the majority of lead is not excreted in the urine at the same rates as in older children (13). Secondly, the amount of lead absorbed is inversely related to chronological age and lead tends to deposit in other tissues such as brain, lung, liver, kidneys, bone, and teeth. In other words, children tend to retain more lead in soft tissues than adults. Even minimal lead exposure can significantly affect neonatal neuronal growth and cause irreversible changes in the preterm brain (1).
The current study displayed that the neonatal BLL change shows a positive significant relationship with neonatal weight. One important aspect of the LBW infant’s physiology is the occurrence of oxidative stress and hypocalcemia. Both can potentiate lead deposition and exacerbate its potential toxicity especially to the growing brain and skeletal system (9, 14).
Multivariate analysis revealed that blood creatinine level after transfusion was the best predictor of BLL% change. This goes in consonance with similar studies which proved that pathologies like acute kidney injury are best predicted by serum creatinine levels which affects up to 20% of critically ill neonates and is associated with an increased risk of mortality (15, 16). On the other hand, another studies argued that although blood creatinine is the most commonly used endogenous marker for GFR, it is not the most adequate marker for the neonatal population. Owing to the physiological characteristics of preterm neonates like low weight, low body mass index, reduced muscular mass, tendency to early renal failure arising from the prematurity itself. Also, GFR is low in fetal and neonatal life (17, 18).
The current study showed that BLL increased in a significant linear fashion after transfusions with a positive significant relationship with lead levels in blood packs. This means that PRBCs had a significant load of lead. All transfusions using these packs delivered a lead amount that exceeded the reference dose (6). Another study also exhibited a direct linear relationship between any lead exposure from the PRBCs transfusion aliquot with the subsequent post-transfusion BLL in the transfused neonate (1).
The current study presented in concordance with previous studies that recognized side effects specific to preterm neonates like the development of BPD, IVH, and ROP may be related to PRBCs transfusions (9). Few studies provided strong proof that receiving blood transfusions is an independent risk factor for the development of the mentioned sequels of prematurity. This is possibly due to the multifactorial essence of these sequels and the reality that small and sick babies are more susceptible to receive blood transfusions (19, 20).
The association between IVH and receiving PRBCs may be related to volutrauma and destruction of the weak blood vessels in the neonatal germinal matrix (21). The BLL percent change was lower in the neonates who suffered these complications. This indicates that those neonates had already a higher BLL before transfusion, thus any change after transfusion was minimal. The high BLL could be due to intrauterine exposure to lead evinced by increased lead level in cord blood samples and preterm delivery (22).
RBC breakdown post-transfusion and the associated oxidative stress increased iron load in blood and was suggested to be one of the causes for the development of ROP and BPD. Neonates with BPD are usually small in size. They require more ventilator assist and blood sampling leading to iatrogenic anemia. Consequently, more blood transfusions would be needed to replace blood removed by sampling (23).
Transfusion-related morbidity in premature neonates might be due to alterations that occur in pediatric PRBCs units like the strengthened level of non-protein-bound iron, heme and oxidative stress during preparation and storage and the confined capability of the premature physiology to tackle such stressors (24).
The significantly higher median BLL% change in neonates who suffered sepsis and those who died within 24 - 48 hours from transfusion indicates that those neonates had already low BLL before transfusion, such that the change after transfusion was high. All transfusion-transmitted infections put neonates at risk, particularly LBWs who already have immature immune systems.
5.1. Conclusions
The study concluded that preterm neonates are at risk of lead exposure hazards due to receiving multiple PRBCs transfusions in the NICU setting. Higher lead levels in PRBCs in our study as compared to previous studies denote exposure of donors to higher lead levels in Egypt and accordingly the recipient preterms, as the study showed a significant positive correlation between infant’s post-transfusion lead levels and the lead levels in the aliquot packets.
5.2. Limitations
The study was on the PRBCs and not the other transfusion products as platelets on assumption that most of the lead load would be from the PRBCs.
Difficulty in measuring urine lead levels doesn’t allow the researches to know the amount of lead that may have been deposited in tissues versus excreted.
5.3. Future Research Implications
BLL screening protocols in blood banks similar to those used during screening for infectious diseases should be implemented. Further studies focusing on the impact of neonatal lead exposure are needed to assess for potential neurodevelopmental impairments in future.