1. Background
Cardiac catheterization (CC) is a mainstay of diagnosis and treatment of many children with congenital and acquired cardiovascular diseases. Due to their invasive nature, both therapeutic and diagnostic CC is associated with vascular risks and complications, including pseudo-aneurysm, arteriovenous fistula, hematoma, transient loss of pulse, thrombosis, and vessel occlusion (1-8). Indeed the most frequent and relevant complications in childhood after CC are vascular injuries (1, 2).
There are several risk factors regarding these complications such as lower weight, longer duration of cannulation, larger catheter diameter, cyanosis and arterial access (6-11).
Vessel thrombosis after CC might jeopardize future diagnostic or interventional cardiac procedures. Physical examination is the initial method to evaluate post catheterization complications at the site of vascular access, but some injuries are subtle and subclinical, which are not recognizable by physical examination; hence, color Doppler ultrasound can be a useful and reliable noninvasive tool for diagnosis and exclusion of vessel complications even in young children (1).
2. Objectives
The aim of this study was to determine prevalence and risk factors of the arterial and venous complications early after CC among children and adolescents via ultrasound.
3. Methods
This prospective study included all diagnostic and interventional CC, performed at Namazi Hospital, affiliated to Shiraz University of Medical Sciences (SUMS), as the main referral center in Southern Iran, April 2016 to April 2017.
We explained the study for the patients or their guardians and informed consent was obtained of them.
The database consisted of case type (diagnostic or interventional), age, weight, gender, diagnosis, site of vascular access, number of tries for vascular access (single try, second or third tries, more than 3 tries are indicated as multiple tries), size and provider of inserted sheaths.
Catheterizations were performed under conscious sedation or general anesthesia based on patient’s condition, but the access points were completely immobile during procedure. Subcutaneous injection of 1% lidocaine without epinephrine was used as local anesthesia. Arterial and venous accesses were obtained percutaneously, using the Seldinger technique [8]. Once the proper position of the wire was confirmed by fluoroscopy, dilator/sheath assembly was gently inserted into the vessel over the wire. For older patients an incision was made with an appropriate size blade. Stepwise pre-dilatation technique was used for larger delivery systems or sheaths. In some neonates, especially for balloon angioplasty of coarctation of the aorta and stenting of the ductus arteriosus, axillary artery was accessed.
Based on the case features various vascular sheath sizes from different manufacturer were used, such as VasCard introducer sheath, Merit Medical Company and Terumo Europe NV.
If we had to use a delivery system more than 2 French bigger than the first sheath, we initially chose a sheath size larger than the first one sequentially, and the proper size delivery system was inserted.
All sheaths and catheters were routinely flushed with one international unit per milliliter of heparinized saline.
Manual compression was applied in all cases following sheath removal by a special nurse. The pressure was enough to stop bleeding and after cessation of bleeding, pressure dressing was carried out. All patients were kept under close observation for any possible complication of vascular access site after catheterization.
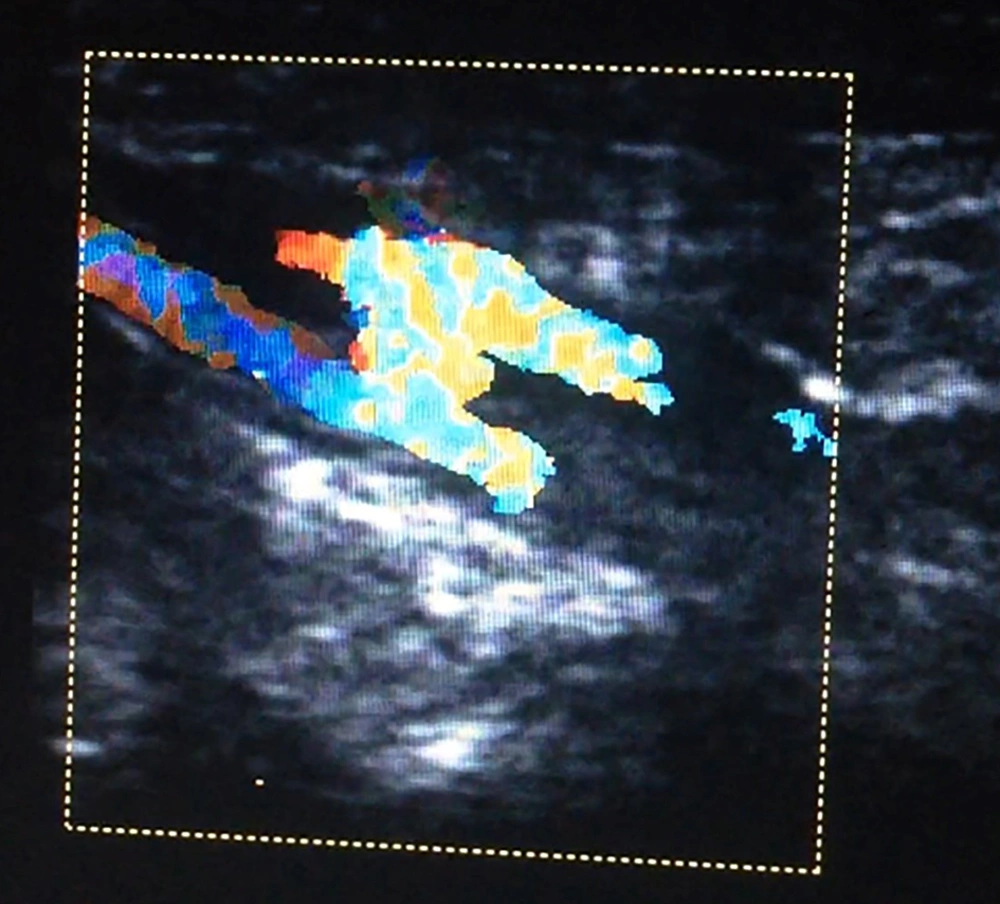
Ultrasound examination was done prior to vascular access in CC laboratory, and post-procedure was done the day after, to rule out any unknown vascular or soft tissue injuries. Before the CC procedure, shape, patency and maximum velocity of the arteries and veins in the site of vascular access were evaluated, and after CC ultrasound examination was done to recognize shape, patency and maximum velocity of the vessels, venous thrombosis, minor or major hematoma, pseudoaneurysm, and any fistula. We extended the ultrasound about 40 millimeters on both sides of the entering points.
Most of the access procedures were guided by ultrasound set especially among patients who were under 10 kilograms, and cases in whom we could not palpate arterial pulses well.
We categorized the arterial complications as more serious, such as dissection, pseudoaneurysm and fistula and less serious, such as acute transient loss of arterial pulse and minor hematoma.
Also venous injuries were stratified as more serious such as major hematoma, pseudoaneurysm and venous thrombosis and less serious such as minor hematoma. Minor hematoma was defined as benign transient hematoma with no need for lab tests or invasive procedures, and major hematoma was defined when a patient developed hypotension or required close monitoring or blood transfusion.
Acute loss of pulse was defined as transient arterial spasm with no serious complication.
Ultrasound examination was performed by an expert sonographer who was not aware of the sheath size and the patients’ conditions. He used a 12-MHz linear ultrasound probe, which included two-dimensional, color and pulsed-wave Doppler modes.
Unfractionated heparin was applied as anticoagulant during CC, and we administered initial bolus of 50 and 100 units/kg for venous or arterial access, respectively. Then, we repeated half of bolus doses every 60 minutes during CC. Prophylactic anticoagulation after procedure was not continued, but aspirin or continuous heparin injection were administered in ductus stenting to prevent stent occlusion.
The entire data after collection was expressed as mean ± standard deviation or number or percentage.
Statistical analyses were performed using IBM SPSS® version 23 and values were considered to be statistically significant when P ≤ 0.05. The independent sample t-test was used to make comparison between the two independent groups and Mann Whitney-U test for nonparametric values and Pearson’s chi-squared test for categorical data to evaluate unpaired data.
4. Results
Totally 173 right or left CCs were performed and 6 patients had more than one arterial or venous access simultaneously. Thus, there were 179 entrance points, and 88 arterial and 91 venous sites were accessed. Of the total, 116 (63.5%) patients underwent both arterial and venous cannulations, besides 33 (19%) cases underwent only arterial, and 30 (17.5%) cases only venous cannulation.
Nearly 70% of the CCs were interventional procedures and 30% were diagnostic (Table 1), and 17 (14%) arterial and 16 (13%) venous access injury occurred while 4% and 5% of them were more serious respectively (Table 2).
| Procedure | Frequency (%) | |
|---|---|---|
| 1 | ASD closure | 8 (6.6) |
| 2 | VSD closure | 5 (4.1) |
| 3 | PDA closure | 28 (23.1) |
| 4 | Balloon valvuloplasty of valvar PS | 10 (8.3) |
| 5 | Balloon valvuloplasty of valvar AS | 2 (1.7) |
| 6 | Balloon angioplasty of CoA | 10 (8.3) |
| 7 | Right diagnostic catheterization | 2 (1.7) |
| 8 | Left diagnostic catheterization | 6 (5) |
| 9 | Right and left catheterization | 40 (33.1) |
| 10 | PDA stenting | 6 (5) |
| 11 | RVOT stenting | 1 (0.8) |
| 12 | CoA stenting | 2 (1.7) |
| 13 | Coronary artery fistula closure | 1 (0.8) |
Types and Frequency of Cardiac Catheterization Procedures
| Access Point Complications | Frequency (%) |
|---|---|
| Venous access complication | |
| No vascular complication | 105 (86.8) |
| Pseudo aneurysm | 3 (2.5) |
| Minor local hematoma | 8 (6) |
| Major local hematoma | 2 (1.7) |
| Venous thrombosis | 3 (2.5) |
| Arterial access complication | |
| No vascular complication | 104 (85.9) |
| Pseudoaneurysm | 2 (1.7) |
| Minor local hematoma | 3 (2.5) |
| Arteriovenous fistula | 1 (0.8) |
| Arterial dissection | 2 (1.7) |
| Transient loss of arterial pulse (arterial spasm) | 9 (7.4) |
Summary of Arterial and Venous Access Point Complications
We stratified the patients in two groups, with and without vascular complications with respect to all complications. The mean age in complicated group was 1.67 ± 1.6 years and amongst without complication was 1.9 ± 3.6 years (P = 0.49), and the mean weight in complicated group was 8.51 ± 8.12 kg and in non-complicated cases it was 9.87 ± 5.9 kg (P = 0.61). Thus, neither age nor weight had significant differences in this group, whereas the incidence of more serious vascular complications was highest among patients younger than 1 year of age, and less than 9 kilograms (87.5%) as shown in Tables 3 and 4.
| Patient No. | Age (y) | Body Weight (kg) | Diagnosis | Sheath Size (F) | Site of Artery | No. of Punctures | Doppler US |
|---|---|---|---|---|---|---|---|
| 1 | 2 | 9 | VSD closure | 5 | Right femoral | 1 | Acute loss of arterial pulse |
| 2 | 0.6 | 5.6 | Rt & Lt catheterization | 5 | Right femoral | 2 | Arterial dissection |
| 3 | 0.25 | 5.2 | CoA Balloon | 5 | Right femoral | 1 | Acute loss of arterial pulse |
| 4 | 2.5 | 11.3 | CoA Balloon | 6 | Right femoral | 1 | Minor local hematoma |
| 5 | 2.5 | 12.5 | Lt heart catheterization | 10 | Right femoral | 1 | Pseudoaneurysm |
| 6 | 0.16 | 3.5 | CoA Balloon | 5 | Right femoral | 2 | Acute loss of arterial pulse |
| 7 | 1.6 | 11 | RVOT stenting | Right femoral | Vein access | Acute loss of arterial pulse | |
| 8 | 12 | 44 | CoA stenting | 10 | Right femoral | 2 | Pseudoaneurysm |
| 9 | 1.2 | 9 | CoA Balloon | 5 | Right femoral | 3 | Minor local hematoma |
| 10 | 0.66 | 7 | PDA closure | 5 | Right femoral | 1 | Acute loss of arterial pulse |
| 11 | 11 | 43 | PDA stenting | 6 | Right femoral | 2 | Minor local hematoma |
| 12 | 0.5 | 6.5 | Lt heart catheterization | 6 | Right femoral | 1 | Acute loss of arterial pulse |
| 13 | 0.16 | 4 | PDA stenting | 5 | Right Axillary | 2 | Arteriovenous fistula |
| 14 | 0.01 | 3 | CoA Balloon | 5 | Right Axillary | 3 | Acute loss of arterial pulse |
| 15 | 0.6 | 5 | PDA closure | 5 | Right femoral | 1 | Acute loss of arterial pulse |
| 16 | 0.66 | 5.6 | Rt & Lt catheterization | 5 | Right femoral | 3 | Acute loss of arterial pulse |
| 17 | 0.01 | 3 | PDA stenting | 5 | left femoral | 3 | Arterial dissection |
Data of the 17 Cases with Post Procedure Arterial Complications
| Age | Weight (kg) | Procedure | Vascular Complication | |
|---|---|---|---|---|
| 1 | 4 months | 5 | PDA stenting | No complication |
| 2 | 2 months | 4 | PDA stenting | AV fistula |
| 3 | < 1 month | 3 | Balloon angioplasty of CoA | Acute Loss of Impulse |
| 4 | < 1 month | 2.9 | PDA stenting | No complication |
| 5 | < 1 month | 3 | PDA stenting | Venous thrombosis (unintentionally) |
| 6 | < 1 month | 2.8 | PDA stenting | No complication |
Axillary Arterial Access and Related Complications
Femoral arteries were preferred as access point in 94.3% followed by right and left axillary arteries in 5.7% 0f the patients. Also, femoral veins were preferred except one case who underwent ASD closure from right jugular vein due to abnormal anatomy of right and left femoral veins (Figure 1). Furthermore, 84.5% of arterial short sheaths were 5 French and 14.5% of the arterial sheaths were 6 French, and we only used one 10 French delivery system for aortic coarctation stenting that became complicated. Diameter of the most introducer short sheaths for venous access was 6 French, while delivery systems were used in 33 cases according to interventional procedures (Table 5).
| Delivery System French | Frequency | Number of Complications | Percent |
|---|---|---|---|
| No need | 84 | 71.2 | |
| 6 F | 8 | 1 (PDA closure) | 6.6 |
| 7 F | 14 | 2 (one PDA closure, one VSD closure) | 11.6 |
| 8 F | 3 | No complication | 2.5 |
| 9 F | 6 | 1 (PDA closure) | 5 |
| 10 or 12 F | 3 | No complication | 2.5 |
Delivery Systems and Associated Venous Complications
One to three puncture tries were done in 61% of arterial and 60% of venous cases and only 12% and 7% of these cases had complications respectively, whereas more than 3 puncture tries were done in the rest of which 23% showed post procedure arterial and 100% venous complications.
Cross tab analysis for sheath size and frequency of arterial and venous complications showed no statistically significant correlation. On the other hand, 3 arterial and 1 venous complication occurred, due to unintentional needle entry into the artery or vein and without insertion of sheath in the associated vessel (Table 6).
| Arterial Sheath Size (F) | Arterial Access Number (%) | Arterial Complications Number (%) | Venous Sheath Size (F) | Venous Access Number (%) | Venous Complications Number (%) |
|---|---|---|---|---|---|
| No (venous access and unintentional arterial access) | Less than 30 (25) | 1 (3) | No (arterial access and unintentional venous access) | Less than 33 (26.5) | 3 (2.4) |
| French 5 | 74 (63.2) | 12 (16) | French 5 | 16 (13) | 3 (2.4) |
| French 6 | 13 (11) | 4 (30) | French 6 | 71 (57.2) | 9 (7) |
| French 10 | 1 (0.8) | 1 (100) | French 7 - 9 | 4 (3.3) | 1 (0.8) |
Relation Between the Sheath Size and Arterial and Venous Access Complications
Seventeen out of 119 patients (14%) with arterial access by means of short sheath and delivery system had vascular injury on Doppler ultrasound, which is demonstrated in Table 3. While 12 procedures were not seriously complicated with acute transient loss of arterial pulse and minor hematoma, 5 patients had complications with more serious injuries including 2 dissections, 2 pseudoaneurysms and 1 fistula. Consequently 4% of them suffered more serious injuries and one of these 5, underwent delivery system insertion (Tables 2 and 3).
Axillary arterial access was done in 6 patients with sheath size 5 F of whom 4 cases were neonates and 2 cases were 2 and 3 months old, while one of them underwent balloon angioplasty of aortic coarctation and rest of them underwent patent ductus arteriosus stenting. Post procedure arterial complications happened for 2 and vein complication for one of the cases unintentionally (Table 4; thus 50% of them developed major or minor complications, and if we subtract axillary access from femoral access injuries, there remain only 9% of femoral arteries that developed minor or major complications.
Sixteen out of 121 patients (13%) with venous access by means of short and long sheaths had some findings on Doppler ultrasound, as demonstrated in Table 4, of whom 8 (6%) patients had complications like non-serious minor hematoma, 2 patients had major hematoma that resolved with packed cell infusion, 3 patients had pseudoaneurysms, and 2 patients developed venous thrombosis due to triple venous lumen insertion after procedure (that was not a direct complication of CC), and 1 patient developed venous thrombosis accidentally because of aortic coarctation ballooning with unintentionally venous needling. Consequently 6 patients (5% of all patients) had more serious venous complications (Tables 2 and 7).
| Patient No. | Age (y) | Body Weight (Kg) | Diagnosis | Sheath Size (F) | Delivery System in Vein | No. of Punctures | Doppler Ultrasound |
|---|---|---|---|---|---|---|---|
| 1 | 0.4 | 8 | PDA closure | 6 | Yes | 1 | Pseudoaneurysm |
| 2 | 0.25 | 5.2 | Balloon CoA | - | No | Arterial access | Vein thrombosis |
| 3 | 1.66 | 6.5 | Rt & Lt catheterization | 6 | No | 1 | Pseudoaneurysm |
| 4 | 0.5 | 7 | Rt & Lt catheterization | 6 | No | 4 | Pseudoaneurysm |
| 5 | 11.5 | 22 | Rt & Lt catheterization | 6 | No | 1 | Minor local hematoma |
| 6 | 0.66 | 5.5 | Rt & Lt catheterization | 6 | No | 1 | Minor local hematoma |
| 7 | 1.66 | 7.3 | Rt & Lt catheterization | 5 | No | 2 | Minor local hematoma |
| 8 | 0.4 | 5 | Balloon PS | 7 | No | 4 | Minor local hematoma |
| 9 | 11 | 34 | PDA closure | 6 | Yes | 1 | Minor local hematoma |
| 10 | 0.75 | 4.5 | PDA closure | 6 | Yes | 2 | Major local hematoma |
| 11 | 0.34 | 8 | VSD closure | 6 | Yes | 1 | Minor local hematoma |
| 12 | 3 d/o | 3 | CoA balloon | - | No | Arterial access | Minor local hematoma |
| 13 | 5 d/o | 3 | PDA stenting | 5 | No | 1 | Vein thrombosis after triple lumen |
| 14 | 0.7 | 9 | PDA closure | 6 | Yes | 1 | Minor local hematoma |
| 15 | 0.66 | 5.2 | Rt & Lt catheterization | - | No | Failed vein access | Major local hematoma |
| 16 | 5 d/o | 3 | CoA balloon | 5 | No | _ | Vein thrombosis after triple lumen |
Data of the 16 Cases with Post Procedure Venous Complications
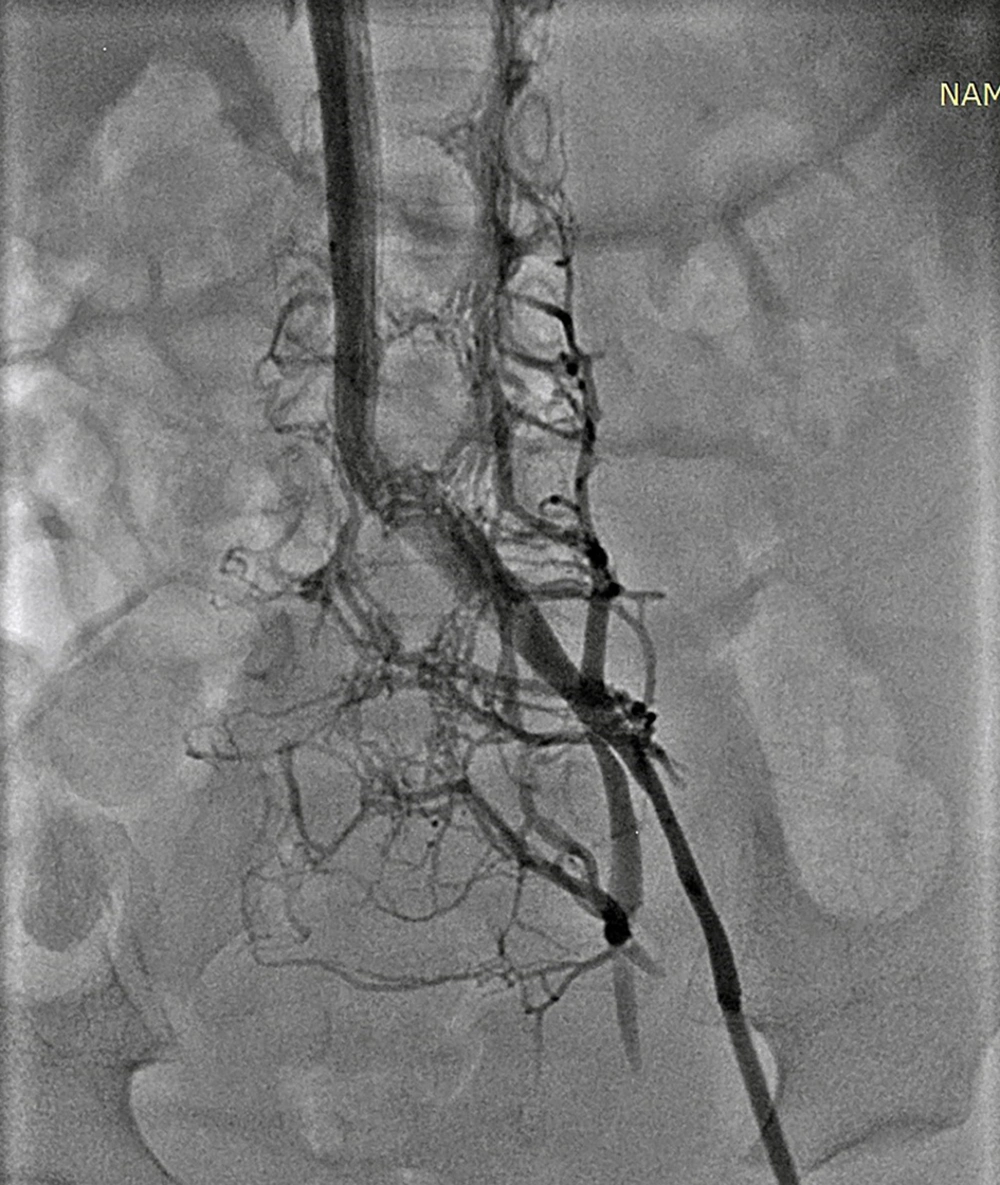
Arteriovenous fistula was a rare complication of CC, and it only happened in one case after PDA stenting via right axillary artery access in a neonate (Figure 2).
The overall incidence of acute loss of pulse was 7.4% (9 patients) and all were transient. Loss of arterial pulses due to transient arterial spasm happened after diagnostic catheterization in 2 patients, and after interventional catheterization in 7 patients. Three patients had aortic coarctation balloon angioplasty, 2 patients had PDA closure with Amplatzer, one patient underwent VSD closure with Amplatzer, and the last one had PDA stenting for pulmonary atresia. In these cases, spontaneous pulsation recovery did not occur and intravenous heparin loading and maintenance infusion over a period of 12 to 24 hours was administered. Pedal pulse recovery was established by clinical examination that was confirmed by the Doppler US examination. None of the patients with arterial and venous complications needed short-term surgical or interventional therapy, although more attention is necessary in the future to avoid particularly arteriovenous fistulas and pseudoaneurysms.
5. Discussion
In this study, we were looking to identify the incidence and factors affecting the development of vascular access complications in children and adolescents undergoing congenital cardiac catheterization.
In our study, the incidence of arterial and venous complications were similar to some previous studies (4, 5), but there were some differences between types of venous complications, such as local hematoma, venous thrombosis and pseudo-aneurysm formation. The most frequent arterial complication was acute loss of pulse due to transient arterial spasm.
In recent studies there were different and heterogeneous reports on complications after CC (2-8). However, most of them have focused on arterial complications (1, 4, 9, 12) and only few studies evaluated and dealt with venous complications (10, 11). Due to increased interventional procedures, it seemed necessary to evaluate CC venous complications more precisely, especially with new introducer sheaths.
While we had no permanent arterial thrombosis, the reported prevalence of arterial thrombosis after CC varied between 0.8% and 40% (6, 9). One study showed that the rate of femoral artery thrombosis in young infants after CC might be underestimated; hence, it is recommended to use Doppler ultrasound after each procedure (1). According to this study and Danish National Patient Registry, routine Doppler ultrasound the day after cardiac catheterization, is recommended in some centers (1, 3) to establish life-long patency of arterial vessel access, and to avoid impaired limb growth. Clearly this recommendation was for arterial thrombosis and there was no suggestion for venous thrombosis.
Some researchers reported that acute occlusive arterial injury was more prevalent in younger children (6, 13), which was in accordance with our study that revealed this inverse relationship for low body weight and arterial and venous access point complications.
The lower age was an influential factor regarding the complications in some researches (6), as in the present study that revealed more prevalence of the serious complications among younger cases.
Several studies showed significant risk differences between interventional and diagnostic catheterizations, in such a way that interventional procedures carried the highest risk (6, 8). In our study, there were no statically significant differences between diagnostic and interventional procedures.
One study on factors affecting vascular access complication in children stated a relatively high incidence of transient loss of pulse after balloon pulmonary valvuloplasty without any attempt to establish an arterial access (8). This unusual complication happened in one of our cases after several attempts for venous access of balloon valvuloplasty of pulmonic stenosis, which might have been due to unwanted entry of the needle into the artery during venous trial.
Arteriovenous fistulas are very rare complications after pediatric CC (14, 15), and fortunately it was resolved by itself without any surgical intervention.
On the other side, 50% of axillary arteries in low weight children, developed major or minor complications, even though they were self-limited, while only 9% of femoral arterial accesses developed minor or major complications.
Some authors evaluated the complications after CC and categorized the severity of adverse events and showed that young age, low body weight and cyanotic or complex congenital heart disease appeared to be risk factors for adverse effects (5, 9). In our study, there was no difference between cyanotic and acyanotic CHD for arterial and venous complications.
5.1. Limitation of the Study
One limitation in our study was paucity of patients and heterogeneity of the procedures, device sizes and profiles; hence, further studies should be performed on more patients.
5.2. Conclusions
Despite great care during vascular access with new low profile equipment, vascular events are still high after CC in children and adolescents, although incidence of serious injuries is low, and venous complications were as high as arterial complications. In summary early detection and long term follow up of these events seems essential, and we might suggest color Doppler ultrasonography after CC in children and adolescents to recognize vascular access point complications.