1. Background
Perinatal period is associated with important cardio respiratory changes from intra uterine to extra uterine life (1). This process can be complicated by severe congenital (2, 3) and systemic diseases (4-7). In advanced stages of some systemic diseases such as asphyxia and sepsis, in neonatal period, cardiac dysfunction has been documented and in other patients exposed to hypoxia because of severe respiratory distress, degrees of myocardial damage has been observed (8-13). In addition to echocardiography that plays an important role in the diagnosis of myocardial dysfunction (14), the use of cardiac biomarkers can improve early diagnosis of cardiac dysfunction and helps timely applying of supportive care (15-17). In very ill neonates, like those with asphyxia, sepsis, or pneumonia, without considering the kind of systemic disease, correlation of serumic troponin I level and myocardial dysfunction can be helpful in the early management without a need to further separating them. Troponin is a cardiac enzyme that has been shown to be a sensitive and specific marker for diagnosis of myocardial damage (15).
2. Methods
This cross-sectional study, after being approved by ethic committees of Hamadan University of Medical Sciences, was performed between December 2016 and December 2018. Term neonates, who were under mechanical ventilation more than 72 hours, were selected from the NICU section of Be’sat and Fatemieh hospitals of Hamadan city. This study was planned to evaluate severely ill patients with systemic disease that underwent mechanical ventilation just to determine correlation of serumic troponin level to echocardiographic myocardial function criteria, thus Apgar score was no relevant variable. Exclusion criteria were premature newborns and neonates with primary cardiac and other congenital anomalies. After taking written consent from their parents, measuring serum troponin I level was performed using ELFA method. Conventional and myocardial tissue Doppler velocities echocardiography evaluations were performed using pulse Doppler with a 2 - 2.5 mm sample volume and 50 to 75 mm depth by Vivid S6 and Mylab60 devices. In conventional echocardiography with transmitral and transtricuspid Doppler study, E (early diastolic) A (late diastolic) wave velocities were evaluated in the diastolic phase of right and left ventricles. The mean values of three heart cycles were recorded. We examined systolic and diastolic function on the left and right side of the heart with the myocardial performance index (Tei index) with (a - b/b) formulation, in which a is the time interval of the closure and opening of atrioventricular valve and b is the ejection time of semilunar valves. Tricuspid plane systolic excoriation (TAPSE) was determined using tricuspid valve M-mode. In the Doppler tissue velocities study of right and left ventricles, Em (early diastolic myocardial wave), Am (late diastolic myocardial wave), Em/Am, E/Em, and Sm (systolic myocardial wave), were measured in three cardiac cycles and the mean values were documented separately. We used another factor for evaluating the diastolic function of the left side of the heart using left atrial ejection force (LAEF) with the following formula: 5 × p × MVA × (peak A velocity) 2 (18, 19), where the constant p denotes the density of blood, the MVA stands for mitral valve surface area and A is the maximum amplitude of A wave in the left atrium ejection phase, which were calculated in the echocardiography (18). The data was analyzed by SPSS-16 software. The descriptive information of qualitative data is presented in table and ratio, and quantitative information as central index and distribution. Normality of data was estimated by Kolmogorov-Smirnov. Non-parametric Spearman correlation coefficient was used to determine the correlation between serum troponin level and the left and the right myocardial function and left atrium ejection force. The confidence level in this study was 95% and the significance level 0.05.
3. Results
Mean serumic troponin I level was 0.213 ± 0.640 and in 18 (30%) patients it exceeded 0.15 ng/mL (150 ng/liter).
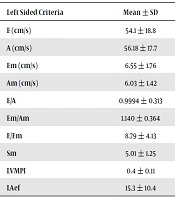
As shown in Table 1, serum level of troponin I was related to echocardiographic E/Em (P = 0.033) and MPI (P = 0.002) findings on the left heart.
| Left Sided Criteria | Mean ± SD | Troponin I (ng/mL), Mean ± SD | Pearson Correlation | P Value |
|---|---|---|---|---|
| E (cm/s) | 54.1 ± 18.8 | 0.213 ± 0.640 | 0.102 | 0.436 |
| A (cm/s) | 56.18 ± 17.7 | 0.213 ± 0.640 | -0.803 | 0.530 |
| Em (cm/s) | 6.55 ± 1.76 | 0.213 ± 0.640 | -0.217 | 0.096 |
| Am (cm/s) | 6.03 ± 1.42 | 0.213 ± 0.640 | 0.013 | 0.921 |
| E/A | 0.9994 ± 0.313 | 0.213 ± 0.640 | 0.209 | 0.110 |
| Em/Am | 1.140 ± 0.364 | 0.213 ± 0.640 | -0.200 | 0.126 |
| E/Em | 8.79 ± 4.13 | 0.213 ± 0.640 | 0.275 | 0.033 |
| Sm | 5.01 ± 1.25 | 0.213 ± 0.640 | 0.205 | 0.115 |
| LVMPI | 0.4 ± 0.11 | 0.213 ± 0.640 | 0.384 | 0.002 |
| LAef | 15.3 ± 10.4 | 0.213 ± 0.640 | -0.116 | 0.377 |
Relation Between Serum Levels of Troponin I and Left Myocardial Function Criteria
In assessing the relationship between the myocardial function criteria of the right heart, E/Em, Sm in tissue Doppler study and TAPSE and RVMPI criteria in conventional echocardiography had a significant correlation with serum troponin I level (P < 0.05) (Table 2).
| Right Sided Criteria | Mean ± SD | Troponin I (ng/mL), Mean ± SD | Pearson Correlation | P Value |
|---|---|---|---|---|
| E (cm/s) | 51.5 ± 12.2 | 0.213 ± 0.640 | 0.185 | 0.157 |
| A (cm/s) | 59.7 ± 13.3 | 0.213 ± 0.640 | 0.075 | 0.567 |
| Em (cm/s) | 5.6 ± 1.75 | 0.213 ± 0.640 | -0.161 | 0.219 |
| Am (cm/s) | 6 ± 1.81 | 0.213 ± 0.640 | 0.062 | 0.640 |
| E/A | 0.87 ± 0.17 | 0.213 ± 0.640 | 0.044 | 0.737 |
| Em/Am | 1.008 ± 0.395 | 0.213 ± 0.640 | -0.209 | 0.108 |
| E/Em | 9.81 ± 2.98 | 0.213 ± 0.640 | 0.379 | 0.003 |
| Sm | 4.9 ± 1.4 | 0.213 ± 0.640 | -0.289 | 0.025 |
| RVMPI | 0.41 ± 0.13 | 0.213 ± 0.640 | 0.358 | 0.005 |
| TAPSE | 6.3 ± 1.36 | 0.213 ± 0.640 | -0.0278 | 0.032 |
Relation Between Serum Levels of Troponin I and Right Myocardial Function Criteria
In 30 patients with TR who could be evaluated completely, a definite inverse relationship was found between the gradient with troponin level (P < 0.001) and E/Em (P = 0.046) criterion on the right side and there was a direct relationship between the gradient and the right indexes of TAPSE (P = 0.007) and RVMPI (P = 0.009). 46.6% of patients had RMPI higher than 0.4 and 45% of patients had LVMPI higher than 0.4. There was no relationship between the gradient and other indices and functional indices of the left ventricle (P > 0.05). Troponin I level in seven (11.6%) patients who received vasopressor (1.14 ± 1.62 ng/mL) was significantly higher than that in 53 (88.4%) patients who didn’t receive vasopressor (0.091 ± 0.171 ng/mL) (P < 0.001).
4. Discussion
The high percentage of respiratory distress results in the use of a mechanical ventilation and hospitalization in the neonatal intensive care unit (5, 6). The use of mechanical ventilation improves oxygenation and reduces CO2 with accurate management of respiratory distress (4, 20). But mechanical ventilation, like other treatments, is not damage-free and can cause short and long term side effects (12). Some of these complications can be tracked and some others can be minimized by taking appropriate measures (10, 14, 21). Although, the recognition of clinical risk factors in early stages can improve the prognosis (12), severe neonatal diseases with high morbidity and mortality rate, can affect myocardial function with different mechanisms (8, 11, 20, 22). Some degrees of cardiac involvement in neonates are associated with an increase in cardiac troponin levels in serum (21, 23), measuring of which can be employed in early diagnosis of cardiac involvement (9). Echocardiographic markers (14, 24, 25) in addition to cardiac biomarkers (16, 17) especially troponin (15, 26-28) can be used to confirm these issues. The exact level of troponin I and other cardiac biomarkers are not clearly determined in neonates (21). Some authors suggest that infants on the neonatal period more likely have higher troponin levels than older pediatric groups without a more severe disease (27). In our study mean serumic troponin I level was 0.213 ± 0.640 (ng/mL) whereas in 30% of the patients its level was > 0.15 ng/mL (150 ng/liter) being > 95% percentile for the age (27). Most studies have shown a relationship between the amount of cardiac troponin levels and myocardial damage due to hypoxia (17) and the reduction of coronary blood flow (15, 16, 29). In our study, the neonates under ventilator had severe and prolonged respiratory distress, and the elevation of troponin I level in serum was related to echocardiographic myocardial dysfunction criteria. In one study, consistent with this study, a significant correlation was found between serum level of cardiac troponin and myocardial dysfunction (9). In the patients with respiratory distress, compared to healthy infants, the average troponin T in sick infants was higher than in normal infants and there was a correlation between troponin T level and the need for use of inotropes and oxygen. In the infants who survived, the duration of ventilation was related to troponin levels (9). The mentioned study concluded that troponin marker may be useful in determining the degree of morbidity in patients with respiratory distress (9). This is similar to findings in asphyxia (30). In the present study, we didn’t study morbidity (neurologic sequels) and mortality rate of the patients. But there was a positive and significant correlation between serum levels of troponin I and the function of right myocardium which was higher than that of left myocardium. Among the left myocardial function criteria, E/Em, LVMPI, and among the right myocardial function criteria, RVMPI, Sm, E/Em and TAPSE had a significant relationship with troponin I level, MPI is a systolic and diastolic shared criterion, E/Em a diastolic criterion and S is a systolic ventricular function criterion. Compared to other studies (31-33) in normal children at this age, in our study the right heart criteria, especially E/Em and Tei indices, were affected more than the other criteria. The MPI and E/Em criteria in our study on both the left and the right heart were related to the troponin level, but compared with the normal neonates, abnormalities of the right heart criteria were more than those of the left side (31, 32). The relationship between these criteria, the level of troponin and the increase of TR gradient indicate that troponin and the right ventricular myocardial function criteria in some patients are likely to be affected by pulmonary hypertension (PH). As pulmonary hypertension affects right ventricular myocardial function (34), myocardial dysfunction in the newborns under mechanical ventilation could be exaggerated by PH distinguished by echocardiography (35). In two patients in our study, who had the highest levels of troponin, PH and abnormalities in right ventricular myocardial function criteria were distinct. It seems that in patients under ventilator and especially those with PH, the increased serum level of troponin I should be taken into consideration. After perception of troponin Importance in asphyxia (36), there are a few studies that show troponins (I and T) increase in congenital heart disease (37) especially when complicated with PH (38). Right ventricular biomechanics in the neonatal period may be effective in cardiac biomarker rising in the right heart problems (39). Right ventricular MPI as an important echocardiographic factor was elevated compared with some previous studies on normal neonates (40), this could be effected by the underlying problem (14). As respiratory and metabolic states of these patients were variable, correlation of single troponin test and metabolic and respiratory acidosis wasn’t possible. The effect of these on serumic troponin level can be evaluated in future studies. Further studies are recommended on patients with PH and other patients especially those who need vasopressor administration in neonatal period.
4.1. Conclusions
In newborns under mechanical ventilation, serum level of troponin I helps to diagnose the right and left myocardial dysfunction. It is more helpful in the presence of pulmonary hypertension.