1. Background
Mechanical ventilation is the highest form of respiratory support in a critical care unit. It is a lifesaving intervention to support the cardiorespiratory status until the underlying disease is cured (1). Nonetheless postoperative mechanical ventilation is required in patients undergoing congenital heart surgery. However, the duration of mechanical ventilation may be extended in some patients. Determining the optimal time to discontinue is usually based on the available clinical and laboratory evidence at the time of extubation and dependent on patient’s ability to sustain adequate gas exchange with spontaneous respiration (2).
It was shown in a previous study that weaning indices were not predictive of extubation success in children. The use of objective criteria with which to predict successful extubation in children could prevent inadvertent premature extubation and the unnecessary prolongation of mechanical ventilation (3).
Inflammation is a normal and expected process following surgery and the intensive care process. However, increased and prolonged inflammation increases the risk of mortality and morbidity. Systemic inflammation reflects the increase in the number of neutrophils in the circulation. The neutrophil lymphocyte ratio (NLR) can be used as a new marker of inflammation in cardiovascular diseases.
2. Objectives
We postulated that NLR might be a readily available and inexpensive objective prognostic index with which to ensure successful extubation after prolonged intubation.
3. Methods
Patients of both genders, aged 0 - 6 years, were included in this study during the immediate postoperative period after correction surgery for congenital heart disease from Agust 2015 to May 2016. All of the study patients received mechanical ventilation on a Siemens® 300A Servo Ventilator at the time of extubation. The physician in the pediatric intensive care unit (PICU) made the decision to wean and extubate, based on each patient’s clinical status, blood gas determination, laboratory determination, and the amount of ventilator support. He also made the decision for reintubation. Particular problems, in terms of cardiac surgery, were not observed in any of the examined patients. Gas exchange parameters, such as partial pressure of oxygen (PaO2 ≥ 60 mmHg), fraction of inspired oxygen (FiO2) ≤ 40%, positive end-expiratory pressure (PEEP) of 7 - 10 cmH2O, and a spontaneous breathing trial (SBT) duration of 20 - 30 minutes, were applied to determine which of the patients could be safely extubated. Post-extubation failure was defined as respiratory distress (tachypnea and accessory respiratory muscle use), hypoxemia (pulse oxygen saturation < 90% and decreased level of consciousness leading to inadequate respiratory efforts), and upper airway obstruction (croup, the inability to clear secretions, cardiovascular insufficiency, and apnea).
Demographic data collected at the time of extubation included the patient’s age, weight, sex, time of extubation and pre-extubation arterial blood gas values. Pre-extubation blood samples were used for the baseline data. Extubation failure was defined as reintubation within 24 hours of extubation for study purposes. Total white blood cell (WBC), neutrophil and lymphocyte count were recorded pre-extubation, and the NLR was calculated.
3.1. Statistical Analysis
Statistical analysis of the data collected from the two outcome groups of successful extubation (SE) and unsuccessful extubation (UE) were compared using SPSS®version 16 for Windows® (SPSS Inc., Chicago, IL, USA). Normally distributed continuous variables were expressed as mean ± standard deviation (SD). Categorical variables were expressed as numbers and percentages. Demographic characteristics, perioperative variables and calculated values were compared using an independent sample t-test, while a comparison was made of the categorical variables using chi-square test.
Correlation was assesed using Pearson’s product-moment correlation coefficient. Receiver operating characteristic (ROC) curve analysis was employed to determine the optimum cut-off levels of the pre-extubated NLR to predict succesful extubation. The odds ratio (OR) and 95% confidence interval (CI) were estimated using different logistic regressions models created to determine the independent predictors of succesful extubation. An attempt was made to identify statistically significant variables in univariate analysis. Multivariate logistic models were created to identify independent predictors of succesful extubation. A P value of < 0.050 was considered to be stastically significant.
3.2. Ethical Approval
The hospital’s research ethics committee approved this study (2016.3 / 2 - 7). Written consent to participate in the study was obtained from the parents of patients hospitalized in the cardiac unit of the PICU in the immediate postoperative period following the surgical correction of congenital heart disease.
4. Results
Ninety-nine patients who had undergone congenital heart surgery were classified into two groups (SE or UE). The UE group comprised patients who required re-intubation within 24 hours. The patient demographics are summarized in Table 1. There were no statistically significant difference between the two groups. The pre-extubation blood test results, recorded gasometric patient values and time of ventilation are shown in Table 2. Statistically significant differences were observed with regard to the blood results of the two groups for WBC (P value 0.001), neutrophil count (P value 0.001), lymphocyte count (P value 0.003), and NLR (P value 0.001), and with respect to the blood gasometric values for PaO2 (P value 0.001), PaCO2 (P value 0.011), and blood lactate levels (P value 0.003).
| Parameters | SE (n = 54) | UE (n = 45) | 95% CI | P |
|---|---|---|---|---|
| Blood pH | 7.39 ± 0.05 | 7.38 ± 0.08 | -6.18 - 4.37 | 0.541a |
| PaO2 | 91.85 ± 23.8 | 73.69 ± 28.9 | 7.65 - 28.7 | 0.001a |
| PaCO2 | 37.87 ± 4.05 | 42.18 ± 10.34 | -7.34 - 1.27 | 0.011a |
| Lactate | 1.83 ± 0.56 | 2.53 ± 1.43 | -1.16 - 0.25 | 0.003a |
| Hematologic parameters | ||||
| White blood cell (103/μL) | 11.59 ± 3.15 | 15.80 ± 3.36 | -5.50 - 2.89 | 0.001a |
| Neutrophil count (103/μL) | 6.79 ± 2.39 | 11.69 ± 3.05 | -5.98 - 3.81 | 0.001a |
| Lymphocyte count (103/μL) | 3.46 ± 1.16 | 2.85 ± 0.96 | 0.17 - 1.03 | 0.007a |
| Platelete count (103/μL) | 258.1 ± 98.9 | 248.6 ± 124.8 | -35.14 - 54.15 | 0.680a |
| CRP | 2.04 ± 1.54 | 2.06 ± 1.03 | -0.55 - 0.51 | 0.937a |
| NLR | 2.08 ± 0.77 | 4.79 ± 2.83 | -3.51 - 1.83 | 0.001a |
| Operative data | ||||
| CPB Time (min) | 108.7 ± 18.3 | 113.4 ± 12.1 | 12.6 - 21.3 | 0.561a |
| ACC (min) | 66.3 ± 22.5 | 72 ± 20.7 | 8.5 - 15.1 | 0.683a |
aIndependent-sample t-test.
Following multivariate logistic regression analysis, the NLR, WBC, neutrophil count, lymphocyte count, PaO2, PaCO2 and blood lactate level remained significant predictors of successful extubation (Table 3).
| Parameters | OR | 95%CI | P |
|---|---|---|---|
| NLR | 8.56 | 1.3 - 11.2 | 0.001 |
| White blood cell (103/μL) | 0.9 | 0.85 - 1.5 | 0.001 |
| Neutrophil count (103/μL) | 1 | 0.8 - 1.3 | 0.001 |
| Lymphocyte count (103/μL) | 0.99 | 0.9 - 1.2 | 0.009 |
| PaO2 | 0.5 | 0.2 - 9.7 | 0.001 |
| PaCO2 | 0.47 | 0.1 - 6.6 | 0.001 |
| Lactate | 1.26 | 0.73 - 5.5 | 0.001 |
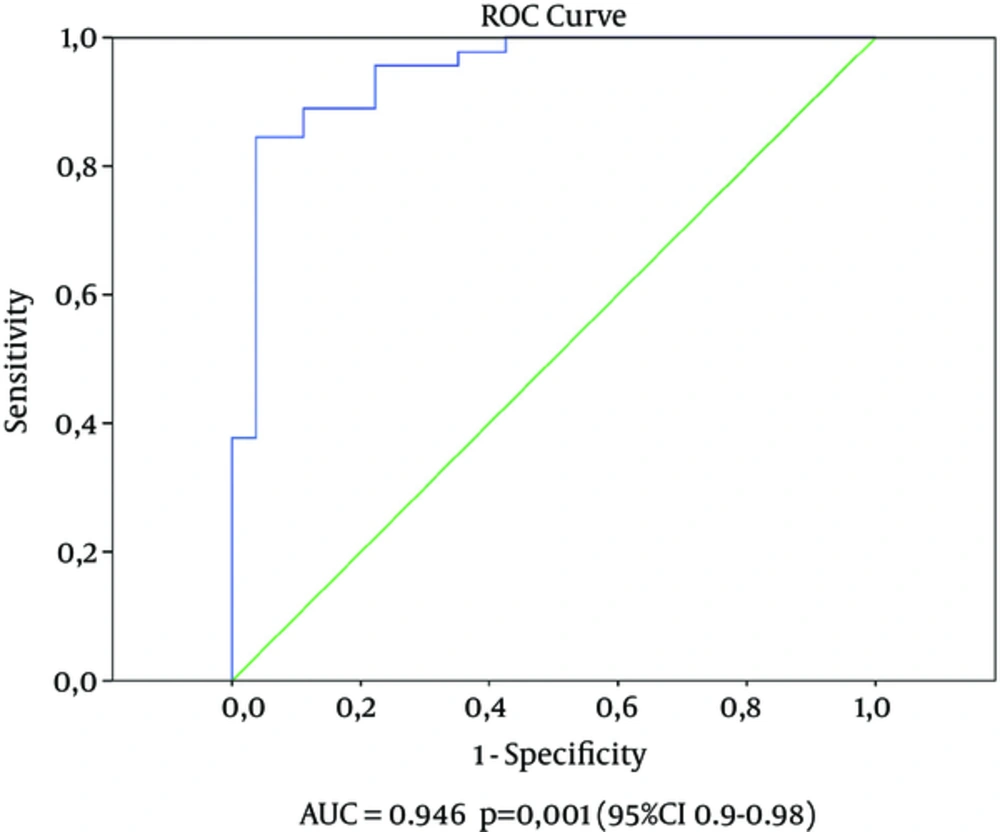
The ROC curves for the NLR were also associated with successful extubation (Figure 1). The area under curve for the pre-extubation was 0.946 (95%CI: 0.90 - 0.98, P value 0.001). Using a cut-off value of 2.65, the pre-extubation NLR predicted successful extubation with a sensitivity of 95% and specificityof 78%. When the study population was divided into two groups using a cut-off value of 2.65, the OR for patients with a NLR ≤ 2.65 was calculated as 11.7 (95% CI: 3.87 - 35.10, P value 0.001).
5. Discussion
The predictive and prognostic value of the NLR has been increasingly investigated and utilized for its prognostic and predictive value (4).
The NLR was found to vary significantly in children in whom extubation failed compared to that in children in whom extubation was successful (following prolonged intubation) and was also predictive of successful extubation in our study.
Pediatric cardiac critical care providers often are challenged with the equally important but often conflicting goals of minimizing patient’s exposure to mechanical ventilation and preventing extubation failure (5, 6). Extubation failures have been associated with adverse outcomes, including increased duration of hospital stay, cardiac arrest and mortality (7, 8). Some authors (9, 10) used a different time period to define extubation failure (< 24 or up to 96 hour after extubation), We evaluated patients who were reintubated within 24 hours as unsuccessful extubation.
The combination of heart disease, surgical stress and having to undergo a cardiopulmonary bypass (CPB) inevitably results in the complex activation and interaction of cellular and humoral mechanisms in patients in an acute inflammatory state. Immunological mechanisms and anatomical characteristics are relatively different in children from those in adults (11, 12). Prolonged mechanical ventilation following pediatric cardiac surgery was consistently associated with longer CPB time in other studies (13, 14). Increased CPB time is required for more complex cases or if unexpected difficulties occur. There is an increased risk of inflammatory response syndrome with generalized edema, decreased respiratory compliance, and coagulopathy with longer CPB time, which decreases the ability to extubate a patient soon after surgery (10). It has been shown in previous studies that prolonged CPB time (≥ 120 minutes) is generally associated with a high risk of failure with regard to weaning from mechanical ventilation (3, 10). Statistical differences were not identified in our study regarding CPB time and aortic cross-clamp time between the two groups.
Lactate levels are known to be a parameter of tissue perfusion and cardiac output, as well as a predictive index of major morbidity after pediatric cardiac surgery (15). In this study, lactate was found to be significantly higher in the UE group than in the SE group. Lactate is an independent predictor of unsuccessful extubation.
Serum PaO2 was significantly different between the two groups, being significantly higher in the UE than in the SE group in our study. Serum PaO2 and serum PaCO2 are independent predictors of successful extubation. Our finding supports the results of a previous study in which it was demonstrated that children requiring long-term mechanic ventilation and with a lower serum PaO2 were associated with weaning failure (16).
Successful extubation in patients is dependent on the resolution of the primary process, the presence of intact airway reflexes, an intact control inspiratory drive, the ability to exchange gases efficiently, and respiratory muscle strength (17, 18).
An accurate prediction of extubation is difficult because multiple factors determine the ability to breathe. However, only a few studies have examined the usefulness of various factors in predicting extubation outcome (19).
“Resistive breathing” refers to a type of breathing exercise that is performed to strengthen the muscles used in respiration (20). The effects of resistive breathing are similar to those involved in the hormonal and inflammatory response to intense physical exercise (21).
It has been indicated in recent experimental and human studies that strenuous resistive breathing, as a form of respiratory muscle exercise, induces proinflammatory cytokines and stimulates the hypothalamic-pituitary-adrenal axis in healthy human (22).
A significant increase in the plasma levels of interleukin IL-6, IL-1β, adrenocorticotropic hormone, and β-endorphin was observed in healthy volunteers following strenuous breathing.
The process of weaning may impose a high inspiratory load on the respiratory muscles, either owing to a small artificial airway size, the accumulation of secretions, or bronchoconstriction during an SBT, or from increased respiratory resistance after extubation because of mucosal swelling and edema that develop in the upper airways, or increased airway secretions and sputum retention, resulting in post-extubation distress. Proinflammatory cytokine production is expected during SBT, contributing to the development of the stress response.
There is recent evidence for weaning-induced cytokine release. Sellares et al examined the levels of IL-6, other cytokines and C-reactive protein, before and at the end of an SBT, in relation to the use of a T-piece in 49 mechanically ventilated patients. They found an increase in IL-6, a major modulator of stress response in SBT failure (23).
Neutrophils play a crucial role in the inflammatory reaction (24). It was suggested in a novel study that neutrophils may have a much more widespread role in immunity and inflammation than what has previously been assumed (25).
Neutrophils are the most abundant WBC. They release various enzymes and cytokines, and activate other immune system cells that trigger and enhance inflammatory reactions (26). After an acute infection or in tissue destruction, the lymphocyte count declines and the neutrophil count rises, leading to an increase in the NLR (27). It was reported in a previous study that both lymphopenia and neutrophilia were independently associated with the severity of infectious disease and the degree of tissue damage (28).
An increased NLR in the postoperative period helps with the assessment of the patient’s immune condition and also provides valuable clues regarding morbidity and mortality. An elevated NLR has been shown to be associated with increased tumor necrosis factor alpha and varying IL levels (IL-6, IL-7, IL-8, IL-12, and IL-17) (29). These markers are known to be associated with a poor outcome in critically ill patients and an increased incidence of recurrent ischemic events in cardiac patients (30). Unfortunately, these biomarkers are expensive and are not routinely performed. By contrast, NLR is measured from a routine blood test, is inexpensive, and simple.
In our opinion, the NLR is a good indicator of inflammatory status. A high NLR, an indicator of the expectation that the inflammatory status of a patient would worsen, accompanied by an increased risk of extubation failure, was accurate in our study, whereas a decreased NLR (a cut-off of 2.65) was predictive of successful extubation, in conjunction with a thorough cardiac and pulmonary examination.
One of the major limitations of our study was that it comprised a relatively low number of cases from a single center.
5.1. Conclusion
Prolonged mechanical ventilation after cardiac surgery increases mortality and morbidity as well as a imposes financial burden on the hospital. So it is very important to determine the most appropriate timing for successful extubation. We believe that the NLR is a very practical and easily measured parameter that can be derived from simple laboratory measurements and which is capable of predicting successful extubation following prolonged intubation in patients undergoing pediatric cardiac surgery.