1. Background
Glypicans are heparan sulfate proteoglycans that are tied to the outer surface of the plasma membrane by a glycosylphosphatidylinositol (GPI) mainstay (1). In general, glypicans, growth factors such as hedgehogs, Wnts, fibroblast growth factors and bone morphogenetic proteins are thought to conduce cellular proliferation and tissue growth by changing cell signaling pathways. In mammals, the glypican family has 6 members (glypican-1 to glypican-6) (2).
Glypican-4 is an adipokine expressed from visceral and subcutaneous adipose tissue. Glypican-4 is an insulin sensitivity enhancer and is associated with body-mass index (BMI) and insulin resistance. Glypican-4 has been shown to be a molecule that enhances insulin signaling by direct interplay with the insulin receptor, independently of the GPI anchorage. This interaction happens with the tenantless insulin receptor and the excitation of the receptor with insulin disrupts the interplay amongst glypican-4 and the insulin receptor. It has also been shown that glypican-4 is important for adipocyte differentiation and increases cellular differentiation. Excess glypican-4 expression or adding of recombinant ectodomain of glypican-4 in produced adipocytes enhances insulin signaling, while glypican-4 depletion decreases insulin receptor phosphorylation and the following downstream signaling (3).
Obesity is an important public health problem worldwide where approximately 25% - 30% of children are affected (4). Studies have shown that 50% of obese adolescents are obese in their adulthood, too (5). Obesity is a chronic proinflammatory condition characterized by the excessive accumulation of lipids and adipose tissue that produce ectopic fat accumulation in different tissues (6). Obesity is associated with dysfunctional adipocytes, cytokine expression at various levels, and increased number of established macrophages in adipose tissue (7). Obesity is the primary reason of insulin resistance in humans and it is the first step to develop type 2 diabetes and metabolic syndrome in many people (8).
2. Objectives
When we investigated the pathogenesis of obesity and the roles of glypican-4, we believed that glypican-4 can have a major act in these patients. The aim of this study was to evaluate the relationship between obesity and serum glypican-4 levels in adolescents.
3. Methods
The current study was confirmed by the Local Ethics Committee (date: 28th August 2018; no: 955) and performed with respect to the procedures of the Declaration of Helsinki.
A total of 80 adolescent patients, aged between 10 and 16 years, were enrolled in our study between September 6th of 2018 and October 29th of 2018. Forty-nine of the study participants were obese patients and 31 of the participants were healthy normal-weight cases. BMI was acquired by partition of the body weight of the patient in kg to the square of the length in meters. According to age, sex and race, adolescents with BMI 95% percentile and over were defined as obese (9). For insulin resistance with the recipe (insulin IU/L × glucose mg/dL/405), homeostatic model of assessment (HOMA-IR) was calculated (10). In obese and control groups, patients with chronic illnesses, smokers, infections, metabolic and endocrinological illnesses, those with malignancy and those who received drug treatments such as corticosteroids, were not included in the study. In addition, adolescents in healthy control group but having insulin resistance were also excluded from the study. The patients who were detected to be in conformity with the inclusion criteria were informed about the research. After taking the informed consent form from the volunteers who wanted to participate in the research, detailed histories of the participants were got. During obtaining blood from the volunteers for their ordinary inspections, only one more biochemistry blood tube was engaged for the study and kept at room temperature for 20 minutes. Then it was centrifuged at 4000 rpm for 10 minutes and the sera was stored at -80°C. During the analysis, the sera were allowed to dissolve at room temperature. The Enzyme-Linked Immuno Sorbent Assay (ELISA) kit was used to measure Glypican-4 levels in serum (Human Glypican-4 ELISA, Elabscience). The minimum finding limit was 0.1 ng/mL. The declared intraassay and interassay variation coefficients (CV’s) were < 4.84% and < 4.06%, respectively.
HbA1c was gauged by high performance liquid chromatography method in autoanalyzer (Biorad, Variant II Turbo, Japan); urea, creatinine, glucose, aspartate amino transferase (AST), alanine amino transferase (ALT), triglyceride tests and total cholesterol were performed by colorimetric method, insulin levels were measured by immune chemiluminescence measures in autoanalyzer (Beckman Brand, AU 5800, USA).
Laboratory tests (glypican-4, HbA1c, glucose, urea, creatinine, AST, ALT, total cholesterol, triglyceride, insulin, HOMA-IR), BMI, sex and age were checked amongst the groups. Correlations between glypican-4 and other laboratory parameters were analyzed in the obese adolescent group.
3.1. Statistical Analyses
IBM SPSS Statistics V. 22 (IBM SPSS, Turkey) programs were performed. The normality of the distribution of the parameters was evaluated by Shapiro-Wilks test. Student t-test was performed to compare the two groups for parameters with normal distribution and Mann-Whitney U test was used to compare the parameters that did not represent normal distribution. Chi Square test and Continuity (Yates) correction were performed to compare the qualitative data. Pearson correlation analysis was performed to investigate the relevance between parameters which were consistent with normal distribution and Spearman’s rho correlation analysis was used to investigate the relevance between parameters that did not comply with normal distribution. Signification was evaluated as P < 0.05.
4. Results
The average age of the adolescents was 13.2 ± 1.8 years. The mean BMI was 27.1 ± 5.1 kg/m2. The study was carried out on 80 volunteer patients, 49 obese adolescents and 31 healthy controls with normal weight. Of the obese adolescents, 41 had insulin resistance, and 8 were not insulin resistant. Demographic and laboratory data of the study groups are summarized in Table 1.
There was no statistically substantial variation amidst the obese and control groups regarding gender distribution, age, urea, creatinine, glucose and total cholesterol levels (P > 0.05).
The levels of glypican-4, BMI, HbA1c, AST, ALT, triglyceride, HOMA-IR and insulin were significantly higher in the obese group than the control group (P < 0.05).
| Obese Adolescent Group (N = 49) | Healthy Control Adolescent Group (N = 31) | P Value | |
|---|---|---|---|
| Age (y) | 12.6 ± 1.8 | 12.8 ± 1.7 | 0.754b |
| Gender | 0.615c | ||
| Female | 21 (42.8) | 15 (48.3) | |
| Male | 28 (57.2) | 16 (51.7) | |
| BMI (kg/m2) | 30.8 ± 3.4 | 24.4 ± 3.5 | < 0.001b, d |
| Glypican-4 (ng/mL) | 1.3 ± 0.4 (1) | 1.1 ± 0.3 (1) | 0.010d, e |
| HbA1c (%) | 5.5 ± 0.3 | 5.3 ± 0.4 | 0.014b, d |
| Glucose (mg/dL) | 90.1 ± 9.2 | 88.7 ± 8.6 | 0.458b |
| Urea (mg/dL) | 25.9 ± 5.9 | 24.7 ± 6.1 | 0.514b |
| Creatinine (mg/dL) | 0.51 ± 0.2 | 0.52 ± 0.1 | 0.736b |
| AST (U/L) | 25 ± 8.6 (24) | 22 ± 5.1 (21) | 0.032d, e |
| ALT (U/L) | 24 ± 12 (20) | 16 ± 8 (14) | < 0.001d, e |
| Total Cholesterol (mg/dL) | 169 ± 34 | 158 ± 39 | 0.136b |
| Triglyceride (mg/dL) | 120 ± 58 (110) | 89 ± 40 (83) | 0.018d, e |
| HOMA-IR | 4.12 ± 2.18 (3.9) | 1.91 ± 0.82 (1.8) | < 0.001d, e |
| Insulin (IU/L) | 19.4 ± 8.1 (15.7) | 8.0 ± 2.94 (6.9) | < 0.001d, e |
Demographic and Laboratory Datum of Obese and Control Groupsa
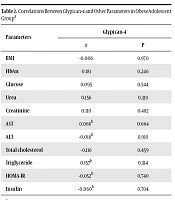
Correlations between glypican-4 and other parameters were evaluated in the obese adolescent group (Table 2). There was no statistically meaningful correlation between glypican-4 levels and BMI, HbA1c, glucose, urea, creatinine, AST, ALT, total cholesterol, triglyceride, HOMA-IR and insulin levels in obese group (P > 0.05).
5. Discussion
Adipose tissue enlargement in obese patients is caused by an increase in lipid storage and differentiation of preadipocytes. A wide variety of autocrine, paracrine and endocrine agents manage adipocyte differentiation (11). Among these, insulin is important in regulating cellular differentiation and lipid accumulation in vitro and in vivo (12). Irregular adipokine secretion from obese children’s expanded adipose tissue contributes to systemic insulin resistance and thus to the development of abnormal glucose metabolism (13). Glypican-4 has been displayed to interact with the insulin receptor to increase insulin sensitivity and to induce differentiation of adipocytes and to act a potentially important role in the regulation of body fat (3, 14).
In this study, serum glypican-4 levels were found to be higher in obese adolescents than normal weighted healthy adolescents. Our study was the first to examine the glypican-4 levels in the adolescent group aged 10 - 16 years. In a study conducted by Leelalertlauw et al., in a group of children aged 8 - 18 years, they found that serum glypican-4 levels increased as the obesity degree increased (15). Li et al. showed that circulating glypican-4 levels increased in prediabetic adults and decreased in patients with type 2 diabetes mellitus (16). They also correlated increased glypican-4 levels with insulin resistance and obesity. Zhu et al. reported increased serum glypican-4 levels in obese adults with insulin resistance (17). Based on this information, high glypican-4 levels in obese adolescents in our study are consistent with the literature.
Glycosylphosphatidylinositol-specific phospholipase D (GPLD1) has been proposed to act as a trimmer in the discharge of glypican-4 from cell surface to circulation (18). This activity of GPLD1 is regulated by insulin (19). Considering the fact that GPLD1 is the most likely candidate to cleave glypican-4, early-onset increases in insulin levels in a prediabetic state may lead to increased glypican-4 release, resulting in increased circulating levels of glypican-4 (20). In our study, elevated insulin levels, which may lead to an increase in GPLD1 activity, may have led to a rising in the glypican-4 levels in the circulation of obese adolescents. We believe that this mechanism is developed to compensate for insulin resistance. In this respect, targeting glypican-4 should be considered as a new approach in the treatment of insulin resistance, obesity and type 2 diabetes.
In their study, Ussar et al. found that glypican-4 levels correlated positively with BMI and insulin resistance, but did not find any correlation with fasting blood glucose and total cholesterol levels (3). Leelalertlauw et al. reported a positive correlation with serum glypican-4 levels and HbA1c, total cholesterol, AST and ALT levels (15). Nevertheless, they did not explore any correlation between serum glypican-4 levels and insulin sensitivity and β-cell function indicators. While Li et al. found a positive correlation between glypican-4 and BMI and HOMA-IR, they reported an inverse relationship with HbA1c and fasting glucose levels (16). Zhu et al. reported a positive correlation of serum glypican-4 levels with BMI, AST, ALT, fasting insulin levels and HOMA-IR (17). Lee et al. found no correlation between glypican-4 levels and BMI or HOMA-IR levels in patients with type 2 diabetes mellitus (21). As can be seen, the literature contains contradictory results about the correlation of glypican-4 levels with other parameters. In current study, we could not detect a statistically significant relationship between glypican-4 levels and BMI, HbA1c, glucose, urea, creatinine, AST, ALT, cholesterol, triglyceride, HOMA-IR or insulin levels in obese adolescent group. This may be explained by the different serum glypican-4 pattern in adolescents. Because obese adults often have decompensated glucose metabolism, while obese children often have an early phase of change in glucose metabolism (15).
5.1. Conclusions
In conclusion, we found high serum glypican-4 levels in obese adolescents. We suggest that in adolescents, glypican-4 levels may be increased in order to reduce insulin resistance in obesity with the aim of compansation.