1. Background
The prevalence of obstructive sleep apnea-hypopnea syndrome (OSAHS) in preschool children ranges from 1% to 3%, which can result in severe complications if untreated (1). OSAHS is defined as a hypoventilation syndrome caused by adenoid tonsil hypertrophy, nasal narrowing, posterior nostril stenosis, cleft palate surgery, tongue hypertrophy, mandibular retraction, small mandibular, laryngeal stenosis, etc. (2). It is often characterized by snoring, difficulty in breathing, paradoxical respiratory movement in the chest and abdomen, apnea, restless sleep, and enuresis at night (3). During the day, OSAHS often causes nasal congestion, mouth breathing, irritability, inability to concentrate, etc. Long-term hypoventilation state and mouth breathing will cause growth and development disorders in various organs of children (4). Studies have reported that OSAHS can cause systemic developmental disorders. In the cardiovascular system, severe OSAHS can lead to pulmonary heart disease and congestive heart failure, as well as left heart failure based on some reports. It has been reported that 37% of children with OSAHS have decreased right ventricular ejection fraction, accompanied by severe cardiac arrhythmiasand hypertension (5). Severe OSAHS, combined with other organ dysfunctions, can lead to developmental disorders (6). OSAHS has severe impacts on neurological cognitive function, manifested as decreased learning ability, inattention, and mental dysfunction in children (7). Long-term mouth breathing in children with OSAHS leads to craniofacial dysplasia, including open jaw, high arch, nasal stenosis, etc. (8). Therefore, early surgical resection therapy for OSAHS is essential for the growth and development of children.
Tonsil adenoidectomy is the most important treatment technology for OSAHS (9). However, the operation-induced throat trauma can cause agitation in children due to pain during the recovery period, accompanied by other symptoms such as unstable vital signs (10). Postoperative agitation is one of the common complications of general anesthesia, which increases the risk of re-bleeding in the operation area, affects the surgical effect, and even causes life-threatening respiratory paralysis and obstruction (11). The traditional care methods we used previously relied heavily on ventilators, ECG monitoring, and general clinical performance of patients. The psychological or physiological agitation of the child and some complications are often overlooked, resulting in slow recovery or increased risk after surgery. Therefore, it is especially important to pay close attention to children’s agitation and respiratory complications during the postoperative recovery period. However, there are a few studies on the treatment and nursing of postoperative agitation and respiratory complications in children with OSAHS.
2. Objectives
In this study, we aimed to investigate the respiratory complications and agitation during anesthesia recovery in children with OSAHS and explored the better-personalized nursing methods to ensure that children smoothly go through the recovery period.
3. Methods
3.1. Demographic and Clinical Data
We selected 200 children diagnosed with OSAHS from June 2014 to June 2017 in the Qingdao Municipal Hospital. The patients were randomly divided into the routine care group (Group A, n = 100) and the personalized nursing group (Group B, n = 100) by computer-generated random number series. The demographic and clinical data of the patients (gender, age, weight, whether noisy in the room) were collected. The family members of all patients signed informed consent forms, and the study was approved by the Ethics Committee of Qingdao Municipal Hospital.
The inclusion criteria included: male or female children aged 2 - 10 years, with no contraindications to surgery, airflow cessation of nose and mouth stopping during sleep is greater than or equal to 10s; apnea plus hypopnea, more than five times per hour, or more than 30 times in seven hours in the evening, and a diagnosis of OSAHS confirmed by polysomnography, based on the 2018 French Association of Otolaryngology (SFORL) guidelines (12).
The exclusion criteria included children aged < 2 or > 10, with developmental disorders, neuromuscular diseases, craniofacial deformity, etc.
3.2. CO2 Laser-Assisted Modified Uvulopalatopharyngoplasty (UPPP)
After entering the recovery room, the vital signs of patients were routinely monitored. Upon general anesthesia, the surgeon, the root of the palatoglossal arch, cuts the mucosa arch to the soft palate in an arc 0.5 cm along the outer edge of the palatoglossal arch. The sacral edge was inwardly cut, and the mucosa at the junction of the pharyngeal arch and the tonsil was cut downward. The tonsil was removed. Sharp peeling of the mucosa and submucosal tissue was performed from the lingual arch, soft palate, and pharyngeal arch. Muscle tissue was preserved. The soft palate part of the proposed resection was cut off, but soft nasopharyngeal mucosa was partly retained. Then, the surgeon grasped the velopharyngeal muscle in the medial 1/3 and pulled it with a 3 - 0 absorbable suture, which was sutured to the genioglossus muscle. Intermittent sutures between muscles were performed. The palatine arch was pulled. The excessive soft palate mucosa was removed, but the mucosa completely covered the wound. After the operation, the pediatric patients were sent to the recovery room by anesthesiologists (13).
3.3. Nursing Methods
3.3.1. Routine Nursing Group
The patients were connected to a ventilator (Siemens Servo-i series ventilator). The relevant parameters were set according to the child’s weight and age. When there was no spontaneous breathing, the breathing mode was set to Intermittent Positive Pressure Ventilation (IPPV), and the IPPV control scheme was pressure control. When there was spontaneous breathing, it was set to Intermittent Forced Ventilation (SIMV). The oxygen flow rate was set to 1~3 L/min. Then, the patients were connected to ECG monitoring quickly and accurately. After extubation by anesthesiologists, the patients’ vital signs, consciousness, complexion, and pharyngeal oozing were closely observed and recorded within one hour after the operation. If no abnormality was found, the children were sent to the general ward by an anesthesia nurse.
3.3.2. Personalized Nursing Group
Patients’ general vital signs were monitored, and breathing was kept smoothly. Postoperative bleeding care: If fresh blood was present after suck due to airway obstruction, or the children vomited fresh blood or blood clots, the surgeon would be immediately notified to check and treat it promptly.
Psychological care: The anesthesia nurse comforted the children through communication and distracted the children’s attention by displaying cartoons, playing with toys, etc., to prevent the children from crying due to pain.
Infusion care: The fluency of infusion was observed when the children were brought into the recovery room after surgery. After checking the amount of fluid infusion in the anesthesia record, the nurses calculated the amount of supplementary infusion per day according to the weight of the child. The nurses should prevent forehead blood circulation from overload due to excessive input in a short period, which would cause several complications such as pulmonary edema or heart failure. The drip rate and temperature were also monitored during fluid infusion.
Pain care: When the children entered the recovery room, the patency of the analgesia pump was observed. The speed of the analgesia pump was adjusted according to the degree of agitation of children to distract children’s attention from pain.
3.4. Observation of Agitation
The agitation of children in the general anesthesia period was mainly defined as a mental state of the separation of consciousness and behavior. The main clinical manifestations were crying, incoherence, and inability to identify things in severe cases (14). The total incidence of agitation was defined as the total number of patients behaving agitation during the whole period of recovery. Counting was not necessary at each time-point.
3.5. Statistical Analysis
All statistical analyses were performed with SPSS software, version 17.0. The counts results are expressed as the number (n) and percentage (%) and analyzed using the chi-square test or Fisher tests. The measurement data are expressed as mean ± standard deviation (mean ± SD), and between-group, comparisons were made using the Student t-test. P < 0.05 was considered statistically significant.
4. Results
4.1. Demographic and Clinical Data of OSAHS Patients
Table 1 shows the demographic and clinical data of OSAHS patients. There was no significant difference in the demographic and clinical data between the routine nursing group (Group A) and the personalized nursing group (Group B) (P > 0.05).
| Group A (n = 100) | Group B (n = 100) | t/χ2 | P Value | |
|---|---|---|---|---|
| Gender (male/female) | 45/55 | 48/52 | 0.181 | 0.671 |
| Age (years) | 5.5 ± 2.4 | 5.3 ± 2.3 | 0.602 | 0.548 |
| Weight (kg) | 34.67 ± 5.92 | 35.71 ± 6.02 | 1.232 | 0.220 |
| Noisy number (cases) | 47 (47%) | 50 (50%) | 0.180 | 0.671 |
at: Student’s t test; χ2: chi-square test
4.2. Incidence of Agitation
The agitation incidence of children in the recovery room was significantly lower in Group B than in Group A in the different periods (all P < 0.05) (Table 2).
| Counts of Agitation (Cases) | χ2 (Chi-Square Test) | P Value | ||
|---|---|---|---|---|
| Group A (n = 100) | Group B (n = 100) | |||
| 15 min | 9 | 2 | 4.714 | 0.030 |
| 30 min | 16 | 5 | 6.438 | 0.011 |
| 45 min | 25 | 8 | 10.491 | 0.001 |
| 60 min | 35 | 15 | 10.671 | 0.001 |
| Total | 45 (45%) | 20 (20%) | 14.251 | 0.000 |
4.3. Incidence of Respiratory Obstruction
The rate of respiratory obstruction was significantly lower in the personalized nursing group (Group B) than in the routine nursing group (Group A) at 15 min, 30 min, and 45 min in the recovery room (all P < 0.05). However, there was no statistical difference between the two groups at 60 min (Table 3).
| Number of Patients with Respiratory Obstruction (Cases) | χ2 (Chi-Square Test) | P Value | ||
|---|---|---|---|---|
| Group A (n = 100) | Group B (n = 100) | |||
| 15 min after surgery | 10 | 1 | 6.157 | 0.005 |
| 30 min after surgery | 8 | 1 | 4.188 | 0.041 |
| 45 min after surgery | 8 | 1 | 4.188 | 0.041 |
| 60 min after surgery | 2 | 0 | 0.497 | |
| Total | 28 (28%) | 3 (3%) | 23.861 | 0.000 |
4.4. Comparison of Vital Signs in the Recovery Room
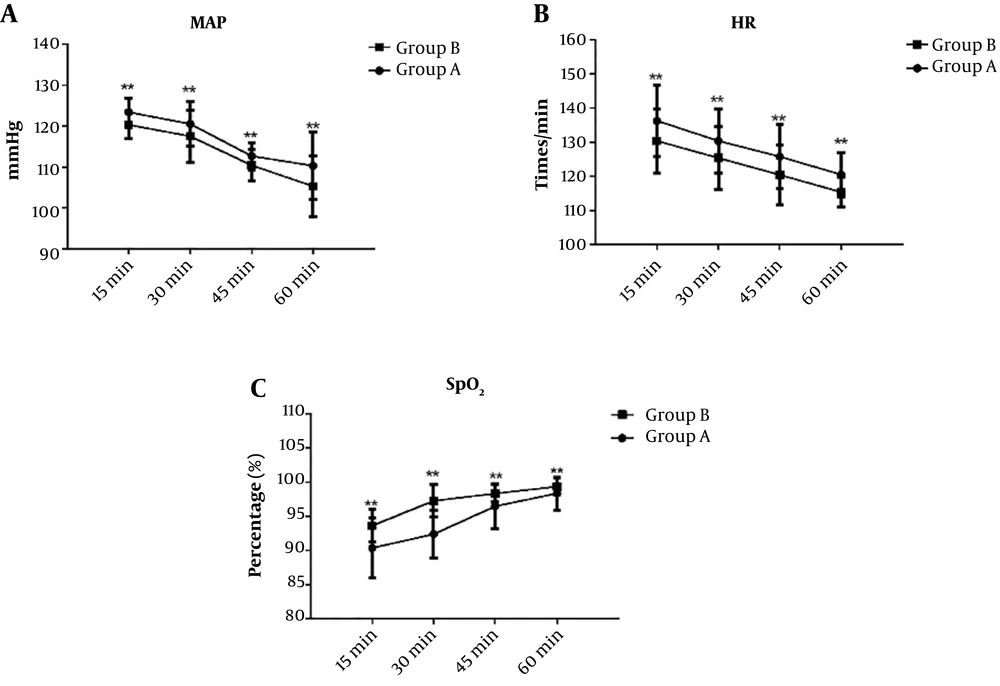
Blood pressure and heart rate were significantly higher in Group A than in Group B at 15 min, 30 min, and 45 min in the recovery room (all P < 0.05), but there was no significant difference at 60 min (P > 0.05). The oxygen saturation of Group A was significantly lower than that of Group B at 15 min, 30 min, and 45 min in the recovery room (all P < 0.05); however, there was no significant difference at 60 min (P > 0.05) (Table 4 and Figure 1).
| Group A (n = 100) | Group B (n = 100) | t (Student’s t test) | P Value | |
|---|---|---|---|---|
| Mean arterial pressure (mmHg) | ||||
| 15 min | 123.42 ± 3.46 | 120.35 ± 3.34 | 6.384 | 0.000 |
| 30 min | 120.58 ± 10.43 | 114.56 ± 8.37 | 3.608 | 0.000 |
| 45 min | 112.72 ± 9.35 | 110.46 ± 8.84 | 4.435 | 0.000 |
| 60 min | 110.34 ± 8.24 | 111.37 ± 7.38 | 0.931 | 0.353 |
| Heart rate (times/min) | ||||
| 15 min | 136.28 ± 10.43 | 130.36 ± 9.37 | 4.223 | 0.000 |
| 30 min | 130.37 ± 9.38 | 125.38 ± 9.21 | 3.800 | 0.000 |
| 45 min | 125.75 ± 9.46 | 120.44 ± 8.84 | 4.101 | 0.000 |
| 60 min | 120.47 ± 6.47 | 119.38 ± 4.37 | 1.396 | 0.164 |
| Oxygen saturation (%) | ||||
| 15 min | 90.37 ± 4.36 | 93.64 ± 2.38 | 6.583 | 0.000 |
| 30 min | 92.38 ± 3.47 | 97.26 ± 2.36 | 11.639 | 0.000 |
| 45 min | 96.46 ± 3.27 | 98.34 ± 1.27 | 5.359 | 0.000 |
| 60 min | 99.06 ± 2.41 | 99.36 ± 1.26 | 1.103 | 0.271 |
Comparison of general vital signs between the two groups of patients after entering the recovery room. (A): Comparison of mean arterial pressure between the two groups; (B): comparison of heart rates between the two groups; (C): comparison of oxygen saturation between the two groups. MAP: mean arterial pressure; HR: heart rate; SpO2: oxygen saturation; Group A: Routine nursing group; Group B: Personalized nursing group. **P < 0.01.
4.5. Hospitalization Time and Expenses
Hospitalization time and expenses were significantly lower in Group B than in Group A (P < 0.001) (Table 5).
| Group A (n = 100) | Group B (n = 100) | t (Student’s t test) | P Value | |
|---|---|---|---|---|
| Hospital stay (days) | 5.23 ± 1.27 | 3.48 ± 1.26 | 9.782 | 0.000 |
| Hospitalization expenses (¥) | 4,568.23 ± 23.47 | 3,628.27 ± 35.28 | 2,221.800 |
5. Discussion
Respiratory first aid events, such as the obstruction of the throat and airways, often occur in the Post-anesthesia Care Unit (PACU). Therefore, the continuous monitoring of vital signs after surgery helps detect and treat respiratory emergencies on time (15, 16). In children with OSAHS, upper airway obstruction, apnea, and arousal usually occur during pre-operative sleep (17). Because the pharyngeal cavity of children with OSAHS is significantly narrower, the airflow quickly passes through the uvula, the base of the tongue, and the epiglottis, resulting in insufficient breathing. Although these children are tried to ventilate, they are still prone to physical activity, turning over, awakening, and swaying because there is no airflow and sound suffocation. When the anesthetic effect ends at the end of the surgery, although the surgery relieves the hypertrophy of the tonsil obstruction, OSAHS children are more likely to experience agitation and respiratory complications due to incisional pain and restless mood. This repetitive cycle destabilizes the vital signs and increases the risk of postoperative wound bleeding in patients. This study found that pain care and postoperative bleeding care can help maintain the patient’s postoperative vital signs and reduce the risk of postoperative bleeding. Psychological care and pain care can effectively prevent postoperative agitation in children with OSAHS after general anesthesia. Therefore, the close observation of vital signs and timely treatment of postoperative respiratory obstruction and bleeding can help ensure the safety of children with OSAHS. Besides, the application of personalized nursing principles can promote postoperative recovery, shorten hospital stay, and reduce hospitalization costs.
Our study found that the rates of respiratory obstruction significantly reduced in children with general anesthesia at 15, 30, and 45 minutes in the recovery room when personalized nursing was performed. The possible reason was that in the first 45 minutes of children’s recovery, the nurse alleviated the pain of children and performed psychological care, which reduced the children’s cry. Moreover, the nurse paid close attention to the respiratory condition of children and immediately relieved the airway obstruction when it happened. Bower et al. report that the close monitoring and timely treatment of respiratory obstruction in children with OSAHS in the recovery room make the children’s vital signs more stable (18). Hasegawa et al. reported that accumulating experience in the prevention and treatment of perioperative airway obstruction in children can effectively respond to such incidents (19).
There are many shortcomings in this study. First, due to the limited time, the number of research samples was small, which may have led to errors in data. The sample size should be further expanded to minimize statistical errors and make the results more convincing. Second, due to the time and financial limitations, the number and objectivity of the test indicators in this study were insufficient. Meanwhile, there was no observation and treatment of postoperative recovery of children with varying degrees of OSAHS. These problems we will improve in subsequent experimental design and research. In addition, we will conduct joint research in multiple regions and use double-blinded methods to verify the results.
In conclusion, personalized nursing during postoperative anesthesia recovery in children with OSAHS can improve the incidence of agitation, reduce the rate of respiratory obstruction, and lower blood pressure and heart rates.