1. Background
Biliary atresia (BA) is a rare progressive inflammatory fibro obliterative cholangiopathy of infancy that affects both extra- and intrahepatic bileducts. If left untreated, this will result in liver fibrosis and cirrhosis and death before the age of two (1, 2). Its reported incidence in the world, varies from 5/100,000 to 32/100,000 live births, and is highest in Asia and the Pacific region. Females are affected slightly more often than males (3).
The BA incidence rate was reported to be 1:14000 and 1:20000 live births in European and North American countries (4-8). In Asian countries such as Japan, its incidence rate was reported to be approximately 1:9000 live births (9). The highest incidence rate was reported from French Polynesia (~1:3000 live births) and Taiwan (1 in 5000) (10, 11).
Although multiple factors including immunologic factors, genetic predisposition, and environmental factors have been related to the pathogenesis of BA, the main cause of BA is not well understood yet (12). Some studies support the role of infections and environmental factor in BA pathogenesis by reporting the seasonal (9) and geographical (13) variations in the incidence of BA. While other studies demonstrated the role of developmental factors in BA pathogenesis by showing the presence of at least one other major anomaly in approximately 20% of patients and also polysplenia syndrome in 8% to 12% of the children with BA (14).
Portoenterostomy and liver transplantation are the two standard treatments for biliary atresia. BA is the most common cause of liver transplantation in children and more than half of the liver transplants were due to BA in childhood (15, 16).
Previous studies conducted in Japan showed that short-term (1 - 3 years) native liver survival (NLS) ranged from 20.3% to 75.8%, ten-year NLS rates ranged from 24% to 52.8%, and 20-year outcome was 27%. Ten-year overall survival (OS) ranged from 66.7% to 89% and 20 years OS was 43% (17, 18). Different factors have been reported as predictors for long-term NLS including age at Kasai portoenterostomy (KPE), anatomical type, accompanying malformation, bridging fibrosis, ductular reaction, ductular size at portahepatis, bilirubin and liver enzymes levels at certain postoperative time point, and aspartate aminotransferase to platelet ratio index (APRI). Rapid clearance of jaundice after KPE is the most important predictor factor for NLS (19-22).
Results of the limited studies on survival rate of BA and its determinants in Iran showed that five-year survival rate was 61% and 14.3% in Shiraz and Tehran, respectively (23, 24).
2. Objectives
Considering the controversies over the long-term outcome of KPE and inadequate information about incidence and outcome of patients with BA in Northwest Iran, we conducted this cohort study in Tabriz Children’s Hospital to evaluate the epidemiological features and long-time outcome in BA patients.
3. Methods
The present retrospective cohort study was conducted in Tabriz Children’s Hospital. This hospital is affiliated with Tabriz University of Medical Sciences and is the referral center for pediatric diseases and one of the major centers performing KPE in Northwest of Iran (an average of 7 cases per year). The patients were referred to this hospital from East-Azerbaijan (capital city: Tabriz), West-Azerbaijan, Ardabil and Kordestan provinces which have cold and mountainous climate. The majority of the inhabitants of these provinces consist of Azeri Turk ethnic group. According to Iran’s census in 2016, the population of these provinces are 10,045,302 and East-Azerbaijan with 3,909,652 population is the most populated province.
We included all BA patients admitted to Tabriz Children’s Hospital from March 2006 to March 2016.
Different methods has been used for BA diagnosis including surgical exploration, intraoperative cholangiography and assessing the pathology of the resection specimen in those who undergo surgical correction. Liver needle biopsy, HIDA scan and ultrasonography were also used for diagnosis.
This study was performed in accordance with the guidelines of the Ethics Committee of Tabriz University of Medical Sciences (IR.TBZMED.REC.1395.539).
3.1. Data Collection
Data was collected through the hospital inpatient/outpatient medical records, the last clinical progress notes and also through contacting patients' parents.
The following data were collected: sex, gestational age, birth weight, presence of congenital malformations, age at referral, age at which jaundice first began to appear, and preoperative and follow-up parameters, such as the age and weight at KPE, post-operative use of antibiotics/ursodeoxycholic acid/corticosteroids, clearance of jaundice, and age at death. Moreover, the collected data include laboratory parameters prior to KPE, such as total serum bilirubin (TSB), direct serum bilirubin (DSB), aspartate transaminase (AST), alanine transaminase (ALT), gamma-glutamyl transferase (GGT), alkaline phosphatase (AP), platelet count, total protein, albumin and prothrombin-time (PT) and AST to platelet ratio index (APRI). APRI was calculated using the formula: [(AST/AST ULN)/platelet count] × 100.
The aspartate aminotransferase to platelet ratio index (APRI) has been proposed as a noninvasive and readily available tool for the assessment of liver fibrosis in chronic liver disease.
Clearance of jaundice was defined as decrease in total bilirubin to less than 2 mg/dL anytime after KPE and successful KPE defined by clearance of jaundice.
Native liver survival rate (NLS) was defined as beginning at birth and continuing to death, liver transplant or last follow-up.
The incidence rate of BA was determined by calculating the annual number of new cases to the number of live births in the same year. Annual number of live births in East Azerbaijan ranged from 62689 to 72489 in our ten years study (http://amar.org.ir/).The incidence rate was only calculated for patients who were originally from East-Azerbaijan.
3.2. Statistical Analysis
All statistical analyses were performed using IBM SPSS Statistics for Windows, version 16. A difference with a P value of less than 0.05 was considered to be statistically significant. The Kolmogorov-Smirnov test was used for assessing the data distribution. Considering the non-normal properties of collected data, nonparametric assumptions were used in statistical analysis. Continuous data was expressed as medians [ranges] and compared with the Mann-Whitney U test. Categorical data were compared with the chi-square or Fisher’s exact test, as appropriate. For survival analysis, we constructed survival curves and tables using the Kaplan-Meier method. Curves were compared with the log-rank test. We used Cox regression analysis to assess the effect of early-life parameters on continued NLS.
4. Results
Between March 2006 and March 2016, 94 BA patients, 50 (53.2%) female and 44 (46.8%) males, have been diagnosed in Tabriz Children’s Hospital. The median age at jaundice onset and admission to hospital was 16.72 ± 5.6 and 62.55 ± 3.53 days, respectively.
About 30% of parents had consanguineous marriage that was significantly higher compared with general population (P = 0.0001).
Acholic stool (pale or clay-colored stools), invisible gall bladder, hepatomegaly, splenomegaly and triangular cord sign (TAS) were seen in 42.5%, 28.4%, 29.2%, 29.5% , and 18.6% of the patients, respectively.
Out of 94 patients, 56 (59.57%) were from East-Azerbaijan, 26 (27.65%) from West-Azerbaijan, 7 (7.5%) from Ardabil, one (1.06%) from Kurdestan, and 4 (4.25%) patients were from the neighboring country, Republic of Azerbaijan.
In East Azerbaijan province an average of 5.6 cases (min 3 cases - max 10 cases) of BA are diagnosed annually.
The incidence rate of BA was 1/13280 live birth in East-Azerbaijan with minimum value of 1/22000 (in 2012) and maximum value of 1/7000 (in 2016) live births. The incidence rate in female and male were 1/11441 and 1/4556, respectively.
Eighty (85.1%) patients had isolated BA, and 14 (14.9%) patients had BA combined with other abnormalities (dextrocardia and hydrocephaly each in two cases, hypothyroidism, Adison's disease, and trisomy 18 each in 1 case, Hirschsprung's disease, duodenal atresia, clivus canal obstruction and frontal bleeding also each in 1 case, CHD in 3 cases). Laterality malformations was observed only in two (2.1%) patients and splenic malformation was not observed in any of our patients.
Out of 94 patients, 33 (33.9%) were born in summer, 23 (25%) in winter, 19 (20.7%) in spring, and 17 (18.4%) in autumn. In BA patients of East Azerbaijan province, summer born patients were 38.9% (21/56) that was statistically (P = 0.04) higher than this ratio in general population which amounts to 27.3% (www.eanocr.ir).
Out of 94 BA patients 71 (75.5%) underwent KPE. The average age at KPE was 64.75 ± 2.47 days. All KPEs were operated in Tabriz Children’s Hospital by three surgeons. Postoperative adjuvant therapies including antibiotics to prevent cholangitis, ursodeoxycholic acid, nutritional support and supplementation of fat-soluble vitamins were the same for all patients.
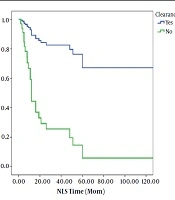
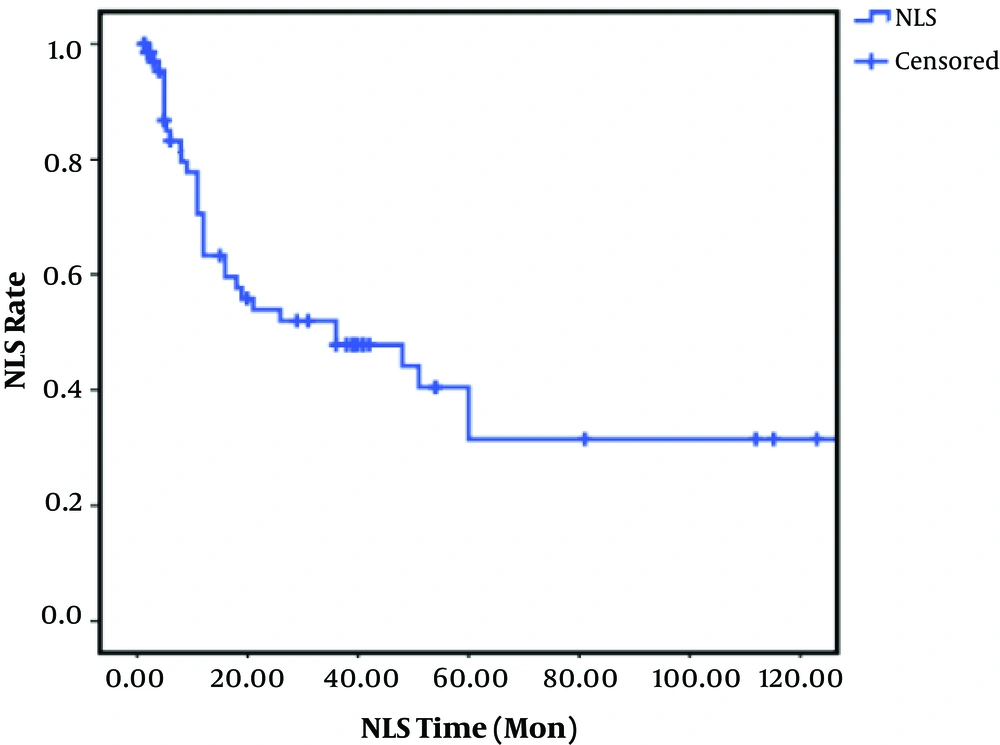
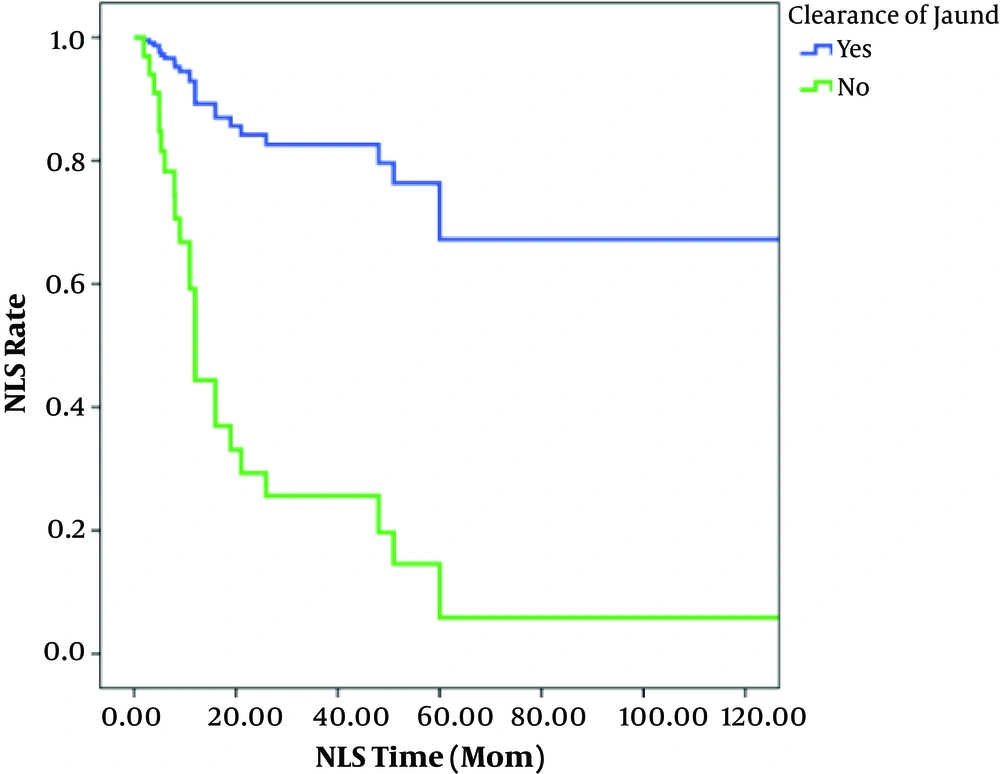
Clearance of jaundice was seen in 42.9% of patients that underwent KPE. In these 71 patients, NLS rates after 1, 2, 3, 5, and 10 years were 73%, 56%, 54%, 40%, and 31%, respectively (Figure 1). In patients with successful KPE, NLS rates at 2, 5, and 10 years of age were 82, 72 and 72%, respectively. In unsuccessful KPE patients, NLS rates were 28, 0.5, and 0.5%, respectively (Figure 2).
Liver transplantation was done in four (5.6%) patients after unsuccessful KPE. Two died one day after the operation, one died in 20 months and one survived with normal liver function. These four patients were younger than two years old at the time of liver transplantation.
The median overall survival rates in our patients were 36 [95%CI: 2.89 - 69.1] months. Univariate analysis showed no significant association between survival rate and gestational age, birth weight, pre-KPE weight, post KPE cholangitis, steroid treatment and age at surgery.
The survival rate of the patients in whom the jaundice cleared was significantly higher than that in those in whom the jaundice did not clear (Table 1). In multivariate analysis clearance of jaundice was highly predictive of survival time. Patients in whom the jaundice did not clear had a nearly 7-fold [HR: 7.25 (95%CI: 1.97 - 26.62), P = 0.003] increased chance of death at any point during the study period, compared to patients in whom the jaundice that did clear (Table 2).
| Hazard Ratio | 95% CI | P Value | |
|---|---|---|---|
| Sex | 0.69 | ||
| Female | 1 | - | |
| Male | 1.146 | 0.57 - 2.28 | |
| Gestational age | 0.96 | ||
| 36 week and more | 1 | - | |
| Less than 36 weeks | 0.970 | 0.22 - 4.12 | |
| Birth weight | 0.51 | ||
| 2500 gram and more | 1 | - | |
| Less than 2500 gram | 1.357 | 0.53 - 3.42 | |
| Consanguineous parents | 0.43 | ||
| Yes | 1 | - | |
| No | 0.777 | 0.41 - 0.45 | |
| Age at surgery | 0.08 | ||
| ≤ 60 | 1 | - | |
| > 60 | 2.021 | 0.90 - 4.50 | |
| Anomalies | 0.59 | ||
| Yes | 1 | - | |
| No | 0.777 | 0.31 - 1.94 | |
| Pre-KPEa weight | 0.83 | ||
| Healthy weight | 1 | - | |
| Under weight | 1.072 | 0.56 - 2.02 | |
| Clearance of jaundice | < 0.001 | ||
| Yes | 1 | - | |
| No | 7.611 | 2.90 - 19.92 | |
| Cholangitis | 0.62 | ||
| Yes | 1 | - | |
| No | 1.220 | 0.55 - 2.70 | |
| Corticosteroids | 0.52 | ||
| Yes | 1 | - | |
| No | 0.713 | 0.25 - 2 | |
| Prophylactic antibiotics | 0.09 | ||
| With 2 antibiotics | 1 | - | |
| With 3 antibiotics | 0.547 | 0.20 - 1.46 | |
| With 1 antibiotic | 0.377 | 0.44 - 8.40 | |
| Prophylactic antibiotics | 0.05 | ||
| Ampicillin/gentamycin | 1 | - | |
| Cefazolin/gentamycin | 0.404 | 0.18 - 1.04 | |
| Others | 0.362 | 0.13 - 0.99 | |
| APRIb | 0.23 | ||
| ≤ 1.66 | 1 | - | |
| > 1.66 | 0.597 | 0.25 - 1.38 |
aKasaei portoenterstomy.
bAST to platelet ratio index.
| Hazard Ratio | 95% CI | P Value | |
|---|---|---|---|
| Age at surgery, days | 0.28 | ||
| ≤ 60 | 1 | ||
| > 60 | 1.699 | 0.64 - 4.45 | |
| Clearance of jaundice | 0.003 | ||
| Yes | 1 | ||
| No | 7.258 | 1.97 - 26.62 | |
| Prophylactic antibiotics | 0.47 | ||
| With 2 antibiotics | 1 | ||
| With 3 antibiotics | 0.521 | 0.13 - 1.95 | |
| With 1 antibiotics | 1.056 | 0.19 - 5.69 | |
| Prophylactic antibiotics | 0.91 | ||
| Ampicillin/gentamycin | 1 | ||
| Cefazolin/gentamycin | 0.962 | 0.30 - 3.03 | |
| Others | 0.729 | 0.36 - 4.27 |
Prior to KPE, there were no significant differences between successful KPE group and failed KPE group regarding clinical and laboratory parameters, except for serum protein and albumin levels (Tables 3 and 4).
| Parameter | Successful KPE | Failed KPE | P Value (Chi-Square) |
|---|---|---|---|
| Sex | 0.2 | ||
| Female | 10 (41.7) | 18 (54.9) | |
| Male | 14 (58.3) | 14 (45.1) | |
| Gestational age | 0.6 | ||
| Less than 36 weeks | 2 (8.7) | 2 (6.9) | |
| 36 week and more | 21 (91.3) | 27 (93.1) | |
| Birth weight | 0.4 | ||
| Less than 2500 gram | 4 (18.2) | 6 (25) | |
| 2500 gram and more | 18 (81.3) | 18 (75) | |
| Consanguineous marriage | 0.2 | ||
| Yes | 4 (17.4) | 9 (36) | |
| No | 17 (73.9) | 16 (54) | |
| Congenital malformation | 0.5 | ||
| Yes | 2 (8.7) | 5 (16.2) | |
| No | 19 (82.6) | 26 (83.8) | |
| Age at onset of jaundice, d | 17.61 ± 28.7 (0 - 120) | 21.16 ± 20.79 (0 - 60) | 0.2 |
| Age at referral, d | 59.89 ± 27.5 (15 - 123) | 57.21 ± 30.81 (6 - 129) | 0.5 |
| APRIb | 0.3 | ||
| ≤ 1.66 | 11 (61.1) | 12 (50) | |
| > 1.66 | 7 (38.9) | 12 (50) | |
| Age KPEc, d | 66.15 ± 30.35 (32 - 139) | 65.87 ± 24.9 (20 - 131) | 0.5 |
| Age at surgery, d | 0.1 | ||
| ≤ 60 | 10 (52.6) | 9 (30) | |
| > 60 | 9 (47.4) | 21 (70) | |
| Pre-KPE weight | 0.1 | ||
| Healthy weight | 7 (46.6) | 12 (53.1) | |
| Under weight | 8 (66.9) | 12 (46.9) | |
| Post-KPE cholangitis | 0.5 | ||
| Yes | 8 (36.4) | 9 (33.3) | |
| No | 14 (63.6) | 18 (66.7) | |
| Post-KPE corticosteroids | 0.4 | ||
| Yes | 2 (11.76) | 3 (10.7) | |
| No | 15 (88.23) | 25 (89.3) | |
| Post-KPE phenobarbital | 0.2 | ||
| Yes | 1 (5.5) | 5 (17.9) | |
| No | 17 (94.5) | 23 (82.1) |
aValues are expressed as No. (%) or mean (range).
bAST to platelet ratio index.
cKasai portoenterstomy.
| Parameter, Pre-KPE | Successful KPE | Failed KPE | P value (Mann-Whitney U Test) |
|---|---|---|---|
| TSB, μmol/L | 11.5 (6.2 - 24.5) | 11.6 (6.1 - 24.5) | 0.8 |
| DSB, μmol/L | 6.5 (1.9 - 10.3) | 6.8 (0.4 - 11) | 0.19 |
| AST, IU/L | 267 (45 - 679) | 226.5 (37 - 463) | 0.4 |
| ALT, IU/L | 129 (25 - 1582) | 131.5 (37 - 463) | 0.15 |
| AP, IU/L | 1207 (295 - 7746) | 1123 (177 - 2931) | 0.1 |
| PT, s | 12 (10.5 - 54) | 12 (10.5 - 23) | 0.4 |
| PTT, s | 40 (28 - 105) | 38 (26 - 82) | 0.3 |
| Total protein, G/dL | 6.5 (5.7 - 7.4) | 5.5 (3.5 - 6.8) | 0.004 |
| Albumin, G/dL | 4.2 (3.6 - 7.8) | 3.7 (2.1 - 4.8) | 0.02 |
| GGT, IU/L | 844 (297 - 1368) | 1054 (227.6 - 1551) | 0.8 |
| APRI | 1.42 (0.24 - 7.43) | 1.29 (0.16 - 36.81) | 0.5 |
Abbreviations: ALT, alanine aminotransferase; AP, alkaline phosphatase; AST, aspartate aminotransferase; DSB, direct serum bilirubin; PT, prothrombin time; PTT, partial thromboplastin time; TSB, total serum bilirubin.
aValues are expressed as mean (range).
5. Discussion
The results of present study showed that the incidence of BA in East-Azerbayjan, Iran was 1/13280 that is lower than that of other Asian countries, including Japan (1:9000) and Taiwan (1:5000) (9, 11) but it is higher than that of European and North American countries (1:14000 - 1:20000) [4-8]. The observed differences in the incidence rate of BA in different countries may be due to the variation in environmental factors.
Several studies described seasonal and geographic variation in the incidence of BA (4, 8, 13, 25-27) and reported conflicting results. In Atlanta, it has been shown that the incidence rate of BA was three times higher in winter compared with spring. However, in New York and Texas, the incidence was higher in autumn (27-30). In contrast to that, in Korea the incidence rate of BA was higher in summer (31). In the present study we also showed that more than one third of all BA patients were born in summer that was significantly higher than that of general population (27.3%) (www.eanocr.ir). This observation may be related to the environmental factors such as timing of possible viral infection around the perinatal period. For example in a study in China, the perinatal infection of Cytomegalovirusis was reported as one of the most important etiologic factors for BA (32). In addition to environmental factor, some studies also suggested the role of ethnicity and genetic factors in the etiology of BA. Previously, it has been shown that the incidence of BA in Asia-Pacific region and multiethnic countries (e.g. New Zealand with 1 in 9181 live births incidence rate of BA) was higher than European countries (33, 34). We also had a higher consanguinity of parents (30%) compared with general population (27%) (35). Some studies also investigated the consanguinity between parents of BA patients and reported conflicting results. In a study conducted in Egypt, consanguinity in parents of BA children was reported to be 10/40 (36). However, in New Zealand among Maori sub-groups, no evidence for higher consanguinity in parents of affected children was reported (37).
There were also other background characteristics of BA patients in the present study. We found a higher incidence rate of BA in females than males. This finding is consistence with the results of previous studies (4, 9, 28-30, 38, 39).
The structural abnormalities were observed in 14.3% of BA patients in the present study that was higher than that of Asian countries (3% - 5%). However, in the western countries the congenital abnormalities are reported in 5% - 14% of BA patients (5, 6, 40). Some disparities were also observed in type of congenital abnormalities between Asian and western countries. In western countries, splenic malformations such as polysplenia or asplenia have been known to be very common where as low rates of spleen anomalies in BA patients were reported from Asian countries (41, 42). Similar to these results, spleen anomalies were not observed in the present study.
We found that the 5-year native liver survival (NLS) rate was 40% in BA patents and this was lower than that of UK (51%), Japan (59.7%), the Netherlands (49%), Taiwan (53%) and Korea (63.4%) (16, 17, 43, 44) and was higher than that of Switzerland (37.4%) (5). Some studies in Iran also showed a 5-year NLS of 14% in Tehran and 61% in Shiraz (23, 24). The observed differences in survival rate between different studies may be related to the differences in age at KPE and the rate of successful KPE. Studies showed that the age of KPE was lower and the rate of successful KPE was higher in countries with higher NLS (16, 17, 39, 43, 44).
Although some studies suggested that combination of BA with another structural abnormality (41, 45), severity of hepatic fibrosis, presence of post KPE cholangitis (46-50), age at KPE (51-53) and adjuvant steroid treatment (54-56) were important prognostic factors determining the rate of a successful KPE, none of the above mentioned factors was associated with higher rate of jaundice clearance in the present study. Similar results of age at KPE (57-62) and adjuvant steroid treatment were also reported in previous studies (54, 55).
This study is the first one that investigated the survival rate of BA patients in Northwest of Iran and the results should be interpreted considering the limitation of the study. One limitation was the small number of patients. However, we studied all BA patients who were referred to the Tabriz Children’s Hospital, the referral center for BA patients in Northwest of Iran. So, the epidemiological features reported in the present study could be representative of BA patients in this region. Another limitation of the present study was the retrospective nature of some of the data. However, we collected the data from the hospital records to avoid information bias.
5.1. Conclusions
In conclusion, we found that the incidence rate of BA in our region was lower than that of other Asian countries. The 5-year NLS rate was 40%. In our patients the biliary atresia was not significantly associated with other structural abnormalities, severity of hepatic fibrosis, presence of post KPE cholangitis, age at KPE and adjuvant steroid treatment. Considering that Iran is a multiethnic country, a multicentre prospective study in different regions of Iran would be recommendable to elucidate the etiopathology and prognostic factors of BA patients in this country.