1. Background
Transient tachypnea of the newborn (TTN) or wet lung is a common physiologic lung disorder characterized by pulmonary edema secondary to clearance delay of fetal alveolar fluid immediately after birth. TTN is a common cause of dyspnea in the newborn. The incidence rates of TTN are 4.0% to 5.7% among term infants and 10.0% in premature infants (1). The risk factors associated with TTN includes; prematurity, male sex, large birth weight, meconium-stained amniotic fluid, cesarian section delivery(esp. elective cesarean), gestational diabetes, maternal chorioamnionitis and maternal asthma (2-5).
TTN is a benign, self-limited clinical condition in most patients but rarely could result in severe complications such as sever hypoxia and death which is called “malignant TTN”. For the management of TTN, it requires cardio-respiratory monitoring; supportive care in the neonatal intensive care unit (NICU) includes: maintaining a neutral thermal environment and providing nutrition, preclude oral feeding, low-percentage supplemental oxygen. Beginning the prophylactic antibiotics coverage is also suggested in literature until blood cultures are reported negative (6-8).
There are few data regarding pharmacotherapy for the management of TTN. Previous studies suggested inhaled epinephrine, oral or intravenous and inhaled furosemide, beta2 agonist and fluid restriction but the most appropriate treatment approach is still matter of controversy (8-10). Randomized controlled trials are needed to confirm the feasibility and safety of the most proper approaches in this field.
We aimed to conduct a randomized clinical trial of the efficacy of inhaled salbutamol for the treatment of TTN. Our primary objective was to assess the effect of salbutamol on major clinical outcomes including duration of oxygen therapy and improvement of respiratory symptoms. Additional analysis focused on the time of initiation of first enteral feeding and duration of hospitalization.
2. Methods
This double blinded randomized clinical trial was conducted from June through December 2014 in three urban tertiary care centers of Babol, North of Iran.
The neonates born 34weeks of gestational age and older who were diagnosed with TTN in the first 6 hours of life were eligible for inclusion in this study. The diagnosis TTN was based on clinical evidence of tachypnea (respiratory rate more than 60 Bpm) with or without cyanosis, respiratory distress (accessory muscle use, nasal flaring, grunting), and chest X-ray findings consistent with TTN (at least one of the radiologic signs which include: lung hyperinflation, perihilar congestion or streaking, fluid filled interalobar fissure, fluffy bilateral infiltration, pulmonary edema). Exclusion criteria consisted of gestational age less than 34 weeks, congenital gross anomalies, neonates born with meconium aspiration, birth trauma or asphyxia, chorioamnionitis, positive history of maternal receiving corticosteroid during 7days before birth, neonatal sepsis (positive blood culture, positive CRP, radiologic findings consistent with pneumonia), persistent pulmonary hypertension of neonate, RDS, neonates with confirmed metabolic disorder (e.g. hypoglycemia, hypokalemia, etc.), neonatal cardiovascular disease (diagnosed with echocardiography).
2.1. Intervention and Data Collection
In total 70 neonates met the inclusion criteria and were randomly assigned to intervention and control group. The intervention group received 0.15 mL/kg (equal to 0.15 mg/kg) inhaled salbutamol (Astalin manufactured by Cipla; India) plus 4 mL normal saline 0.9% by nebulizer within 10 minutes. Placebo group received 0.15 mL/kg normal saline 0.9% plus 4 mL normal saline 0.9% by nebulizer within 10 minutes (11). At the admission blood samples were collected of all the neonates for evaluation of blood biochemistry and electrolytes, CBC, CRP, and Arterial Blood Gases. A chest X-ray was taken. Enteral feeding was discontinued due to tachypnea and respiratory distress. After stabilization of clinical status and resolving of distress the enteral feeding began and the time recorded. Standard fluid administration of 60 mL/kg/day on the 1st day of life for term neonates and 80 mL/kg/day for preterm neonates was performed.
Before and after treatment in intervals of 30 minutes, one, four and six hours the respiratory rate, oxygen saturation, oxygen requirement, retraction score (Silverman-Anderson retraction score) was evaluated and recorded (11). Major study outcomes included total duration of respiratory support or oxygen therapy, the time of initiation of enteral feeding and duration of hospital stay which were recorded for each patient.
2.2. Data Analysis
Statistical analysis was performed using SPSS version 18 (Chicago, USA). Independent t-test and paired t-test were used for quantitative variables as mean (SD) such as: gestational age, birth weight, maternal age, Apgar score, pH, respiratory rate, heart rate, retraction score, FiO2, O2 saturation, retraction score, duration of oxygen therapy, first enteral feeding and duration of hospitalization. Chi-square test was used when the data from a variable presented as n (%) such as: sex, mode of delivery, maternal medical history. Repeated measures analysis was done for evaluating the trend 1 of change for quantitative variables in each group during the study period. P values less than 0.05 were considered statistically significant.
3. Results
In total 70 neonates (45, 64.3% males and 25, 35.7% females) who met the inclusion criteria of the study were randomly assigned in either salbutamol (35) or placebo (35) group. The mean gestational age was 257 ± 13.2 days, mean birth weight 3000 ± 693 grams. Mean age of the mothers 27.8 ± 6.1 years (Table 1). There was no significant difference in gestational age, birth weight, maternal age, parity, gender ratio, mode of delivery and maternal past medical history between the two groups. Also there was no significant difference in 5-minutes Apgar score, arterial pH and the onset time of respiratory symptom between salbutamol and placebo group.
| Variables | Group | P Value | |
|---|---|---|---|
| Control | Salbutamo | ||
| Gestational age, day | 256.7 ± 12 | 258.2 ± 14 | 0.65 |
| Birth weight, gr | 2988.3 ± 720 | 2988.3 ± 720 | 0.88 |
| Maternal age, y | 28.8 ± 6.9 | 26.8 ± 5.1 | 0.17 |
| Parity, n | 1.86 ± 0.87 | 1.69 ± 0.71 | 0.37 |
| Sex | 0.80 | ||
| Male | 23 (65.7) | 22(62.9) | |
| Female | 12 (34.3) | 13(37.1) | |
| Mode of delivery | 0.84 | ||
| Vaginal | 3 (8.6) | 2 (5.7) | |
| Elective cesarean | 19 (54.3) | 21 (60) | |
| Emergency cesarean | 13 (37.1) | 12 (34.3) | |
| Maternal medical history | 0.87 | ||
| Gestational diabetes | 1 (2.9) | 4 (11.4) | |
| Hypothyroidism | 0 (0) | 1 (2.9) | |
| Preeclampsia | 1 (2.9) | 1 (2.9) | |
| Preeclampsia/Hypothyroidism | 1 (2.9) | 0 (0) | |
| Gestational diabetes/Hypothyroidism | 1 (2.9) | 0 | |
| Negative history | 31 (88.6) | 29 (82.9) | |
| Apgar score 5th, min | 8.89 ± 0.32 | 8.71 ± 0.57 | 0.12 |
| Arterial pH | 7.34 ± 0.055 | 7.34 ± 0.059 | 0.73 |
| Respiratory symptom initiation, min | 47.3 ± 66.8 | 29.4 ± 49.6 | 0.2 |
Demographic Findings of Study Population with TTN Between Placebo and Salbutamol Group (N = 35)a
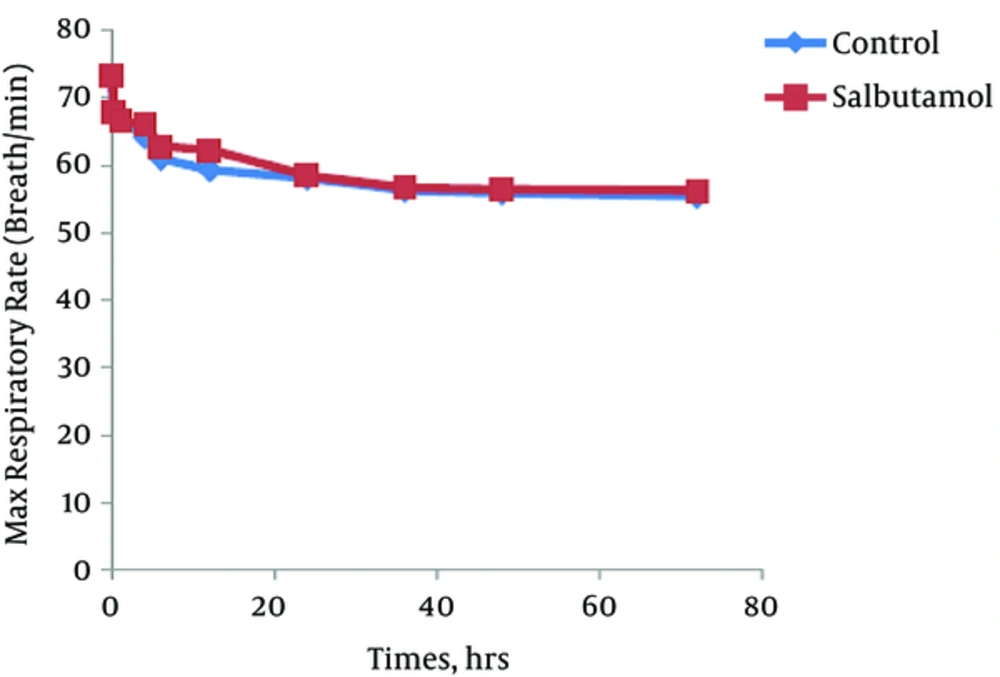
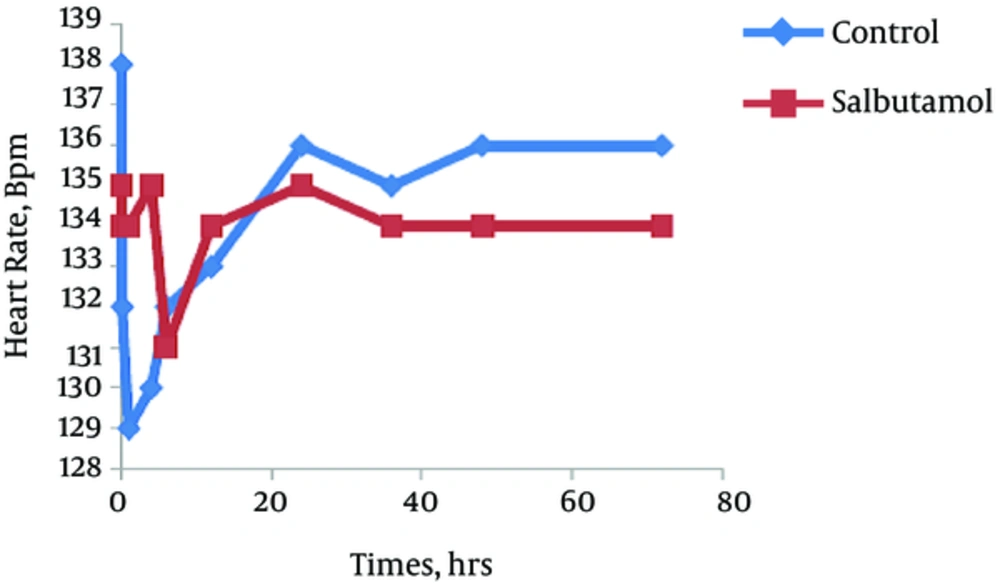
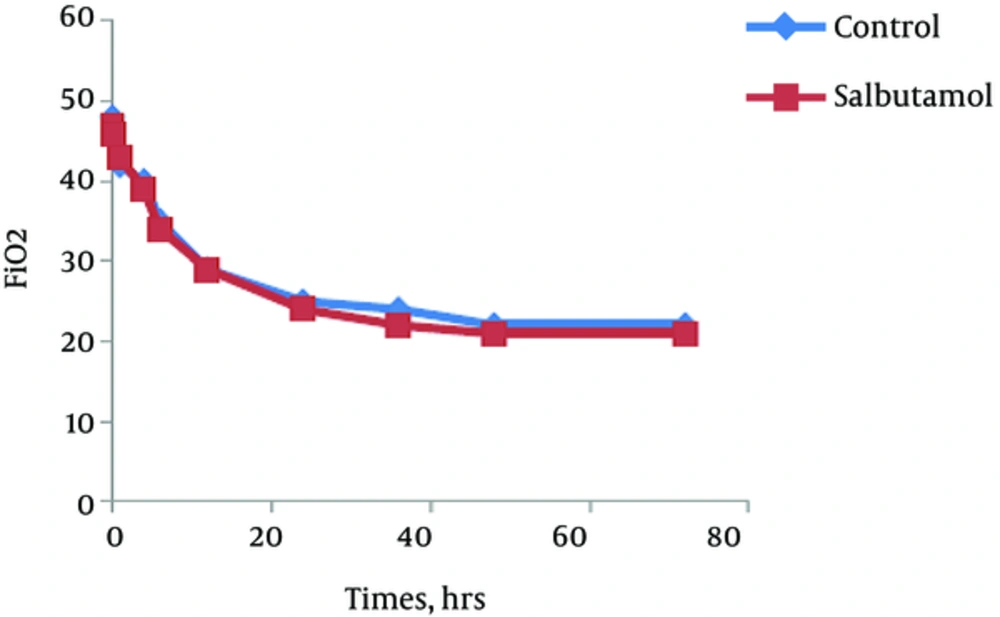
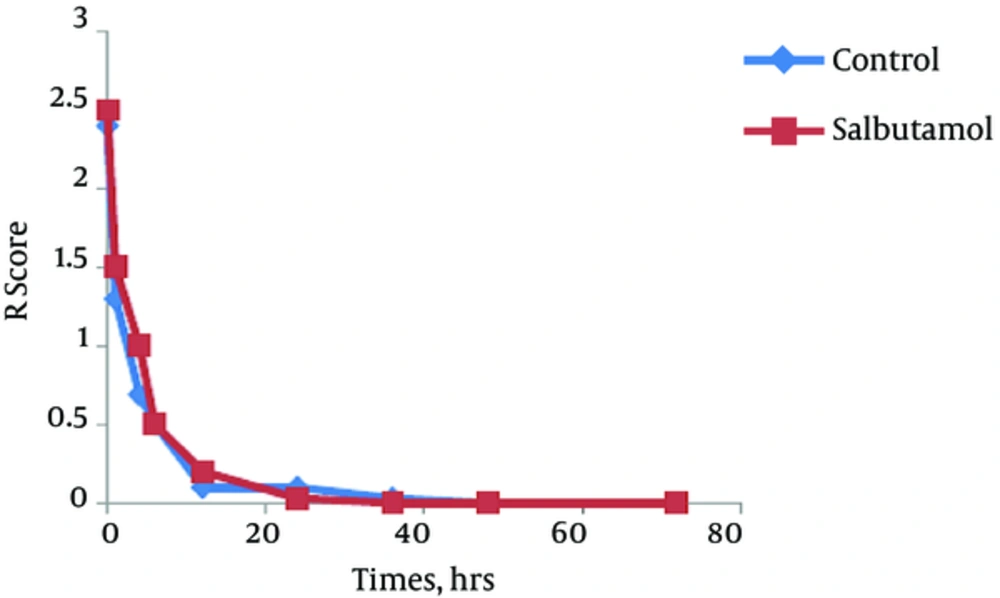
Independent t-test revealed no significant difference between the clinical findings of salbutamol and placebo group before and 24 hours after the initiation of the study (Table 2). The mean values of respiratory rate, heart rate, FiO2, retraction score, oxygen saturation, enteral feeding initiation, duration of oxygen therapy and hospitalization was not different between groups. But based on paired t-test analysis the mean values of the respiratory rate, FiO2 and retraction score in before and 24 hours after the initiation of the study was significantly decreased in both groups (P < 0.001).
| Variables | Group | P Value | |
|---|---|---|---|
| Control | Salbutamol | ||
| Respiratory rate, Breaths/min | |||
| Before treatment | 72.8 ± 10.9 | 73.2 ± 13 | 0.87 |
| 24 hrs after treatment | 61.76 ± 15.75 | 58.5 ± 9.2 | 0.83 |
| P valueb | < 0.001 | < 0.001 | |
| TTN clinical score | |||
| Before treatment | 2.4 ± 1.3 | 2.5 ± 1 | 0.62 |
| 24 hrs after treatment | 0.48 ± 1.41 | 0.04 ± 0.2 | 0.27 |
| P valueb | < 0.001 | < 0.001 | |
| Heart rate, Beats/min | |||
| Before treatment | 138.9 ± 13.7 | 135.9 ± 18.5 | 0.45 |
| 24 hrs after treatment | 135.9 ± 12.1 | 133.4 ± 9.5 | 0.89 |
| P valueb | 0.36 | 1.0 | |
| FiO2, % | |||
| Before treatment | 48.7 ± 4.4 | 47.7 ± 5.3 | 0.39 |
| 24 hrs after treatment | 25.8 ± 10.9 | 24.4 ± 8.1 | 0.51 |
| P valueb | < 0.001 | < 0.001 | |
| O2 saturation, % | |||
| Before treatment | 97.6 ± 2 | 97.3 ± 1.8 | 0.47 |
| 24 hrs after treatment | 97.6 ± 1.9 | 98 ± 1.6 | 0.32 |
| P valueb | 0.06 | 0.88 | |
| Oxygen therapy duration, hrs | 26 ± 29.3 | 18.7 ± 12.5 | 0.18 |
| Enteral feeding initiation, hrs | 36.8 ± 27.8 | 28.9 ± 10.1 | 0.12 |
| Duration of hospitalization, days | 5.4 ± 2.4 | 4.8 ± 1.2 | 0.19 |
Comparison of Clinical Findings of Neonates with TTN Before and 24 Hours After Treatment Between Placebo and Salbutamol Group (N = 25)a
The trend of the changes during 72 hours of the study for respiratory rate, heart rate, FiO2 and retraction score are displayed in Figures 1 - 4. There was significant decrease during the study period among each group in respiratory rate, FiO2 and retraction score (P < 0.001).
After exclusion of the neonates with retraction silverman anderson scor < 2, fifty patients remained. In the salbutamol group the mean values of primary outcomes including duration of oxygen therapy (P = 0.04), duration of hospitalization (P = 0.006) and initiation of enteral feeding (P = 0.013) were significantly lower than in placebo group (Table 3).
| Variables | Group | P Value | |
|---|---|---|---|
| Control | Salbutamol | ||
| Oxygen therapy duration, hrs | 32.72 ± 35.6 | 17.12 ± 9.5 | 0.04 |
| Enteral feeding initiation, hrs | 42.6 ± 31.6 | 25.9 ± 7.49 | 0.013 |
| Duration of hospitalization, days | 6.24 ± 2.43 | 4.68 ± 1.06 | 0.006 |
The Comparison of Primary Outcomes After Exclusion of the Neonates with R Score Less Than 2 (N = 25)
4. Discussion
The primary objective in our study was to evaluate the efficacy of inhaled salbutamol on major clinical outcomes of TTN patients to determine whether inhaled salbutamol could affect duration of respiratory support, length of hospitalization and initiation time of enteral feeding. According to our findings, single dose inhaled salbutamol has no significant effect on primary objectives when compared to placebo group in our 70 neonates study population. To further analyze the efficacy of salbutamol treatment, we excluded the patients with retraction silverman anderson scor less than 2 and re-analyzed the data in remaining 50 patients. Significant difference was observed between the 2 groups, in the duration of respiratory support (P = 0.004) and hospitalization (P = 0.006) and the time that enteral feeding initiated (P = 0.013). These findings suggest that salbutamol inhalation therapy may be helpful for the treatment of the newborns with more critical condition.
Although the clear physiopathology of TTN has not been understood yet but the potential therapy for TTN must be based on an understanding of the mechanism of normal fetal lung fluid clearance at birth. Previous literature suggested that ineffective clearance of fluid and malfunctions of pulmonary epithelial ion transport processes in fetal lung are major mechanisms for this pathology (12, 13). Mechanical force of birth canal and Starling forces seem to have partially contribution to this process and fluid clearance is mainly mediated by the activation of transepithelial sodium reabsorption through the amiloride-sensitive epithelial sodium channels (ENaC) and sodium-potassium adenosine triphosphate ( Na+-K+-ATPase) activity. Therefore, the disruption of this chain due to inadequate Na+ transport, either because of decreased numbers of transporters or inactivation can lead to retention of fluid in alveolar space (14, 15). Beta2 adrenergic (beta2A) receptors are present throughout the lung, including the alveolar airspace, and play a pivotal role for regulation of the active Na+ transport in ENaC and Na-K-ATPase (16). Experimental studies documented the therapeutic role for the beta2 agonist in the treatment of pulmonary edema by accelerating the clearance of excessive fluid from the alveolar space by increasing the function of epithelial transport proteins (17, 18).
Despite the common use of beta2A in treatment of neonatal respiratory illnesses and chronic lung disease in premature infants, limited studies investigated the dosage, duration and efficacy of administering inhaled beta2A in the management of neonatal respiratory disease (19, 20). Recent studies conducted to investigate the efficacy of intravenous administration of albuterol (salbutamol), a beta2A, on pulmonary edema models both in-vitro and in-vivo in adult patients, supported the evidences that beta2A could be an efficient pharmacological intervention in patients with acute respiratory distress syndrome (21, 22). On the other hand, the positive protective effect of beta2A receptor signaling on alveolar active Na transport in normal and injured lung provides substantial support for the use of beta2A agonist to accelerate alveolar clearance in TTN (16).
In a similar study, Armangil et al. revealed that single dose inhaled salbutamol treatment was effective with respect to both clinical and laboratory findings without adverse events (11). In comparison to their study we did not utilize the advanced respiratory support such as CPAP and mechanical ventilation for our population except for two cases in control group who were intubated and ventilated mechanically due to spontaneous pneumothorax about 23 and 27 hours after initiation of oxygen supplementation therapy with hood transfusion. On the other hand, the major difference between these studies was the mean primary retraction score in neonates which was 2 - 3 in our study population according to Silverman-Anderson retraction scoring system and 7 - 8 in Armangil et al. (whose study was based on TTN clinical score of Respiratory Distress Assessment Instrument scoring system). At the second stage analysis, by exclusion of the retraction scores less than 2 we accomplished our primary objective compatible to the similar studies. In a study in Korea, Myo-Jing Kim et al. indicated that the duration of supplemental oxygen therapy and empiric antibiotic therapy were significantly shorter in the salbutamol group but there was no significant difference in duration of tachypnea before 72 hours of admission and duration of hospitalization (23).
Similar to other studies, none of the neonates in intervention group developed complications including tachycardia or arrhythmias after administrating the inhaled salbutamol. It seems that salbutamol therapy could be a low-risk candidate for the management of TTN patients.
Esengul Keles et al. have shown that inhaled β-adrenergic agonist added to humidified oxygen was found to improve clinical and laboratory parameters of neonate with TTN (24).
We encountered several limitations in our study. As multicenter study, the study population was not large enough to reach a high power. But in contrast to similar RCT in a row we had the larger number. We did not classify the study population based on the type of delivery and we gathered the population as the same group. It is suggested that in future studies researchers differentiate infants by the method of delivery. On the other hand, for obtaining better evaluation of the management efficacy, we suggest that neonates with low TTN clinical symptoms could be excluded from the study.
4.1. Conclusion
Our randomized-controlled trial indicated that inhaled salbutamol could result in shorter duration of respiratory support and hospitalization and earlier initiation of enteral feeding in TTN patients with moderate to severe respiratory symptoms without exposing to any adverse effects during follow up.