1. Background
Down syndrome (DS) or trisomy 21 is the most common type of trisomy among children (1, 2). Children with Down syndrome, in addition to the moderate mental retardation, are susceptible to congenital heart disease and other organ diseases that may be affected by genetic spectrum in these patients (3, 4). Coincidence of CHD (congenital heart defect) and other organ diseases can influence prognosis and mortality rate (5, 6). Approximately half of these patients have congenital heart disease (1, 7-9) and usually require cardiac surgery, which has a good prognosis (10, 11), but other organ involvements (12, 13) such as leukemia may worsen the prognosis of the cardiac treatment (14, 15). Detection of risk factors that increase this association can be important in the management of Down syndrome (16, 17). Based on the results of some studies, radiation exposure may increase the risk of leukemia incidence (18). Down syndrome patients are prone to leukemia more than the normal population. High dose radiation that is used in the congenital heart disease angiography and intervention may increase leukemia risk in these patients (19, 20).
2. Methods
This descriptive-analytic study was performed on children with Down syndrome in Neonatal, Pediatrics, Pediatric Cardiology and Hematology Departments in the city of Hamadan between 2010 and 2017, after extracted from the paper and electronic records. Inclusion criterion was Down syndrome confirmed by karyotype and the existence of the patients’ medical records in the hospital archives. Exclusion criteria were the flawed data recorded in the patients’ records and the impossibility of eliminating defects. After completion, the data was analyzed by SPSS-16 software. Central and dispersion indices were used to summarize quantitative variables. To compare the risk of leukemia in children with and without congenital heart disease, and patients with and without angiographic history, the odds ratio (OR) was used. All analyzes were performed at 95% confidence interval and the level of significance was considered less than 0.05.
3. Results
A total number of 118 cases were enrolled to the study from 2010 to 2017. In terms of gender, 60 (50.8%) patients were male and 58 (49.2%) were female. The mean and standard deviation of the age of the children with Down syndrome were 2.78 ±4.29 (range 16 days to 25 years old). Fifty two (44.1%) cases were under one year and 11 (9.3%) above ten years old. 98 (83%) cases (49 males and 49 females) had CHD and 20 (16.9%) cases didn’t have CHD (9 females (15.5%), 11 males (18.3%) (P = 0.684). Of 98 cases with CHD, 92 cases (77.96%) had only congenital heart disease; and 6 (5.08%) cases had leukemia in addition to congenital heart disease (Table 1). From 8 leukemia cases, 3 (5.2%) were males and 5 (8.3%) females, these differences were not statistically significant (P = 0.495). 2 cases (1.68%) had only leukemia and 18 cases didn’t have any evidence of CHD and leukemia.
| CHD | Leukemia | ||
|---|---|---|---|
| No | Yes | Total | |
| No | 18 (15.5) | 2 (1.7) | 20 (17) |
| Yes | 92 (78) | 6 (5.1) | 98 (83) |
| Total | 110 (100) | 8 (6.8) | 118 (100) |
Abbreviation: CHD, congenital heart disease.
aValues are express as No. (%).
bOdds ratio (OR) = 1.70 (95% confidence interval between 0.110 and 3.14).
As shown in Table 1, in patients with co-morbidity of Down syndrome and leukemia, the odds ratio (OR) was 1.70 (95% confidence interval between 0.110 and 3.14).
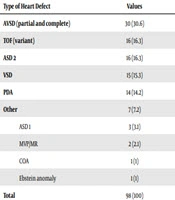
The incidence of CHD was 83% in hospitalized Down syndrome. Different types of congenital heart disease are summarized in Table 2. Eleven patients had pulmonary vascular obstructive disease or Eisenmenger syndrome (ES) that included complete atrioventricular septal defect (AVSD) and large ventricular septal defect (VSD) and large patent ductus arteriosus (PDA). Three ES patients were border-line for diagnosis of primary pulmonary hypertension. Because some echocardiography reports didn’t support tetralogy of Fallot (TOF) diagnosis in the TOF/AVSD category based on pulmonary stenosis gradient and anatomy, we had to put them in the AVSD group. In terms of type of leukemia, 7 cases (87.5%) were ALL and 1 case (12.5%) was AML.
| Type of Heart Defect | Values |
|---|---|
| AVSD (partial and complete) | 30 (30.6) |
| TOF (variant) | 16 (16.3) |
| ASD 2 | 16 (16.3) |
| VSD | 15 (15.3) |
| PDA | 14 (14.2) |
| Other | 7 (7.2) |
| ASD 1 | 3 (3.1) |
| MVP/MR | 2 (2.1) |
| COA | 1 (1) |
| Ebstein anomaly | 1 (1) |
| Total | 98 (100) |
Abbreviations: ASD, atrial septal defect; ASD 1, ASD primum; ASD 2, ASD secundum; AVSD, atrio-ventricular septal defect; CHD, congenital heart disease; CoA, coarctation of Aorta; DS, down syndrome; MVP/MR, mitral valve prolapse/mitral regurgitation; PDA, patent ductus arteriosus; ToF, tetrallogy of Fallot; VSD, ventricular septal defect.
aValues are expressed as No. (%).
Of 98 patients examined with CHD, 49 (50%) had not taken any intervention for their illness. In 33 (33.68%) patients’ angiography and interventional catheterization and in 16 (32.26%) cases surgical treatment were done. Interventional angiography was performed in the 8 cases of which seven cases had PDA device closure and 1 case had atrial septal defect (ASD) device closure.
Among 98 patients with CHD, leukemia developed in six cases and angiography was done in four of them (Table 3) and two cases didn’t have history of angiography. From 4 patients with leukemia and history of angiography, 3 patients had VSD and one patients had large PDA, two of whom died after corrected heart surgery (VSD) and interventional angiography (PDA device closure) because of leukemia (under chemotherapy), one patient (VSD) died before heart surgery. The last leukemic patient had large VSD with ES who is under chemotherapy and had two times angiography. One case developed leukemia in the first year after angiography and two cases developed leukemia after two years and the fourth case developed leukemia after 3 years of angiography.
| Procedure | Type of Disease | |||
|---|---|---|---|---|
| CHD with Leukemia | CHD Without Leukemia | Total | OR | |
| With history of angiography (group 1) | 4 | 33 | 37 | 3.93; 95% CI: 0.686 - 22.63 |
| Without history of angiography (group 2) | 2 | 65 | 67 | |
| Total | 6 | 98 | 104 | |
Abbreviation: CHD, congenital heart disease.
Based on telephone calls, of 118 studied patients, 43 (36.4%) cases died according to their parents’ statements, including 5 patients with CHD and leukemia and one patient with pure leukemia. Thirty six cases of DS with CHD died without documented causes of death, of whom 12 cases had TOF, 15 cases had complete AVSD, 5 cases had large VSD and pulmonary hypertension (PH), 3cases had large PDA with severe PH, 1 case had Ebsteins anomaly, 9 cases had Eisenmenger syndrome and primary pulmonary hypertension (PPH), and 4 cases had history of surgery. 10 patients who died, had history of angiography.
4. Discussion
Down syndrome or trisomy 21 is the most common type of trisomy which is seen as a regular trisomy in 95% of cases and as chromosomal displacement or mosaicism in the other 5% (1, 2). Due to the presence of exogenous gene materials on chromosome 21, this syndrome is associated with several malformations, including congenital heart problems and blood disorders such as leukemia (3, 4). One of the main causes of death in children with Down syndrome is congenital heart disease. The coincidence of Down syndrome with heart disease increases the risk of death (1, 5, 6). The incidence of congenital heart disease in the general population is about 0.8%, but in Down syndrome it reaches about 40 to 60% (1, 7). The incidence of congenital heart disease in the present study was higher than those reported in other studies (7-9, 21). This finding was seen because of stable hospitalized patients at admission were included in this study, and if the patients were more stable (not hospitalized), were omitted. Unfortunately 36.4% of DS were died because of poor follow up by parents, their family or health care services, based on telephone calls.
In the present study, AVSD was the most common congenital heart anomaly in Down syndrome patients (41.8%), which is consistent with older studies (1). In a few later studies (7, 8), ASD and VSD were more common than AVSD and one study showed the increasing prevalence of ASD and VSD was associated with decreased prevalence of AVSD (9). Since genetic spectrum of congenital heart disease in Down syndrome is different (4), the prevalence of different types of CHD in Down syndrome may not be the same. AVSD is divided into two main groups, partial and complete AVSD. The latter group can move toward the irreversible pulmonary vascular obstructive disease or ES in the first year of life. In this study, 11 patients were diagnosed as inoperable stage (Eisenmenger syndrome) indicating delayed referral or treatment. In this study, TOF category was the most common cyanotic CHD. Unfortunately the most patients in this group didn’t have good follow up and management so they died (based on telephone call reports). With early medical and surgical interventions, the prognosis of congenital heart disease in Down syndrome is improving (10). In spite of this, the prognoses of different types of CHD in Down syndrome are not the same and isolated simple CHD has a better prognosis than complex or multiple CHD, for example, AVSD (11). On the one hand, identifying the other associations in Down syndrome can be effective in the overall prognosis of the disease (12, 13). In a big case-control study 2117 patients with acute lymphoblastic leukemia and 605 patients with acute myelogenous leukemia were compared to a healthy sample through telephone interview. The results of the study showed that there is more congenital anomaly in ALL patients compared to control group. The OR (odds ratio) of association of Down syndrome and CHD was calculated 4.85 and 1.48, respectively. On the other hand, when congenital abnormalities occur in children with Down syndrome, makes them susceptible to a higher risk of leukemia compared to normal population (13). In addition, some studies showed that the genetic pattern of Down syndrome renders them susceptible to leukemia (22). This association can lead to poor prognosis (13, 14). However these patients require chemotherapy, possible cardiotoxity of which can probably be intensified in these patients (15, 16). In this study, 1.68% had only leukemia and 5.3% of cases had congenital heart disease and leukemia at the same time, 3 (50%) of whom died after treatment of CHD because of leukemia.
In the present study, the risk of having leukemia (odds ratio) in patients with congenital heart disease was 1.7 in comparison to patients without congenital heart disease. In patients with history of angiography it was 2.79 in comparison to patients without the history of angiography. In the present study, of 98 patients with CHD, six patients had leukemia with CHD and 2 had leukemia without CHD. Given that both Down syndrome (16, 17) and radiation (18, 19) separately increase the risk of leukemia, it is possible that the synchronization of these two factors will exacerbate the risk of leukemia (20) This increase in risk for both CHD (4) and leukemia may be related to the genetic pattern of Down syndrome (22). Some environmental factors such as high radiation doses (18, 19), may increase incidence of leukemia in these patients. In congenital heart disease, angiography and interventional catheterization may use high doses of radiation that is important in this issue. In our study, despite the fact that in patients with CHD, in comparison to patients undergoing angiography and patients who were not under angiography, the incidence of leukemia was not significantly different (P = 0.078), this increase in risk in the group of patients with CHD who underwent angiography may be clinically important. The risk for leukemia in Down syndrome was 6.8%, in Down syndrome with CHD it was 6.1% and this risk for those patients who had history of angiography was 12.1%. One study reported overall risk for association of leukemia in Down syndrome to be 2.1 to 2.7% (20) that was less than that of the present study. With an increase in the number of samples in multicenter studies, this increase in risk can be studied more accurately. In this study, 36 cases died without documented causes of whom 10 cases had history of angiography. This may be important but it was based on information given with telephone call by their parents, so it may be inaccurate.
4.1. Study Limitation
Because this study was a retrospective study based on patients’ files, some demographic data was unavailable and because in some patients, angiography and interventions were performed in other centres, some data, for example, time of angiography and radiation dosage were not determined.
4.2. Conclusions
Coincidence of Down syndrome with congenital heart disease and history of angiography might be seen with leukemia and this may be clinically important.