1. Background
Azithromycin has anti-inflamatory and immune-modulatory properties. Azithromycin reduces various pathophysiological inflammatory responses in cystic fibrosis (CF) lung (1-5). Pseudomonas aeruginosa (PA), the main pathogen contributing to progressive lung impairment in CF, is intrinsically resistant to azithromycin. In this regard, azithromycin is not used for the treatment of CF pulmonary infections with this pathogen. However, azithromycin exerts antivirulence effects on PA, like inhibiting biofilm production and quorum sensing of these bacteria (1, 6-9).
In line with the above mentioned advantages of azithromycin, results of clinical trials have elucidated beneficial effects of azithromycin in CF lung disease in several endpoints including improved forced expiratory volume in one second (FEV1), reduced respiratory exacerbations, need for additional antibiotics and increased weight in patients both chronically infected with PA and uninfected with PA as well (10-12). A Cochrane database systematic review showed that 6 months treatment with azithromycin improves respiratory function in patients with CF and diminishes the frequency of pulmonary exacerbations (13).
Based on the above evidence, the Cystic fibrosis foundation guideline and the American Pulmonary Clinical Practice Guidelines currently advocate routine use of oral azithromycin for all patients with CF aged above 6 years irrespective of chronic PA infection. Oral azithromycin is used as anti-inflammatory therapy to ameliorate lung function and reduce exacerbations for a period of 6 - 12 months (1, 14).
Other promising advantages of chronic (lasting 6 - 12 months) azithromycin use in patients with CF includes decreased acquisition rate of the pathogenic bacteria; non-tuberculous mycobacteria, Methicillin resistant Staphylococcus aureus and Burkholderia cepacia complex (15).
However, use of oral macrolides in CF has been debated to induce acquired resistance of S. aureus and Haemophilus influenza (1, 13, 16). In addition duration of treatment with oral azithromycin remains to be elucidated. Data on efficacy and safety of longer treatment duration or courses of periodic administration of azithromycin is limited (13, 17-19). Studies have demonstrated that beyond the first year of treatment with oral azithromycin, the beneficial effects decline and drug related problems ensue (1, 17-19). A recent study by Samson et al., illustrated decreased efficacy and increased rate of macrolide resistance after 1 year of therapy (19). Another concern with oral azithromycin is the caution required for torsades de pointes arrhythmias (1). Increased QTc intervals after oral azithromycin initiation has been observed in adolescent males, leading to monitoring OTc interval being suggested throughout the course of chronic azithromycin treatment (20, 21).
Delivery of azithromycin by inhalation achieves high drug levels in the airway (22) and presents an attractive alternative to its oral administration.
2. Objectives
Switching to nebulized form may counter many limitations of chronic oral azithromycin use for this life limiting disease. However, to the authors’ knowledge there is no trial to evaluate the efficacy and safety of nebulized azithromycin in the treatment of lung disease in children with CF.
3. Methods
3.1. Study Setting and Participants
This study was performed in the CF center at the Children’s Medical Center, Tehran University of Medical Sciences (Tehran, Iran). Patients aged 8 - 18 years with diagnosis of CF with FEV1 25% - 75% predicted were included. Diagnosis of CF was made by a documented sweat chloride test. All the patients in CF clinic are registered in Iran’s CF registry. Participants were required to be clinically stable and have chronic P. aeruginosa colonization.
Exclusion criteria were: (1) current use of corticosteroids; (2) lung transplantation; (3) recent changes in antimicrobial, bronchodilators, anti-inflammatory medications or chest physiotherapy program; (4) sputum positive for non-tuberculosis mycobacteria; (5) within 28 days (to screening) administration of oral, intravenous or inhaled antipseudomonal antibiotics and azithromycin.
Before participating in this study, eligible patients were required to have a washout period of oral azithromycin for at least 2 months and 28 days for inhaled aminoglycoside.
This study was conducted in accordance with the Declaration of Helsinki and approved by Tehran University of Medical Sciences’ Ethics Committee. Written informed consent was obtained from the participants or their parents. This study was registered in IRCT.IR; (IRCT2016100930233N1).
3.2. Study Design
This study was a prospective, randomized, open-label trial. The random allocation schedule was permuted block randomization technique. Patients and treating physicians were not blinded to treatment allocation (open-label design). Participants were randomly assigned to receive either nebulized azithromycin 70 mg/day (intervention arm) or oral azithromycin 3 times per week (control arm) for a duration of 28 days. Oral azithromycin dose was 500 mg in patients above 40 kg and 250 mg for those below 40 kg according to CF guideline (14). Other treatment modalities during the trial were bronchodilators, hypertonic saline and daily physiotherapy in both treatment arms. During the study period, participants did not receive inhalation and oral chronic suppressive therapy of Pseudomonas aeruginosa in order to evaluate the effects of azithromycin on this pathogen colonization characteristics.
Azithromycin solution for nebulization was prepared from intravenous vials containing 500 mg lyophilized powder by Grupo Tecnimede, Portugal. First, each lyophilized powder vial was prepared with 5 cc normal saline according to the manufacturer’s instruction. For nebulization of azithromycin, 70 mg dose was drawn from the prepared vials, then diluted in 4 cc of 0.9% sodium chloride and was administered by Omron jet nebulizer. According to the manufacturer’s declaration, prepared azithromycin vial is physicochemically stable for one week when refrigerated (2ºC - 8ºC). So, each vial was consumed for one week for each patient.
All nebulized medications were administered with 30 minutes interval; salbutamol was administered first, followed by hypertonic saline, chest physiotherapy and nebulized azithromycin.
Primary outcome included efficacy which was assessed by mean changes in FEV1 percent predicted from baseline (study entry; day +1) to the end of trial (day +28). Secondary outcomes were changes in Pseudomonas aeruginosa colonization characteristics (count, phenotype), quality of life, weight and body mass index (BMI) change and adverse effects. Sputum was analyzed for P. aeruginosa characteristics at the same time points. Quality of life was also assessed at baseline and end of study by PedsQL-4 questionnaire and patient self-reports as well. Safety of nebulized azithromycin was assessed in the first administration and then based on patients’ reports. First nebulized azithromycin dose was administered at CF clinic to monitor for intolerance (test dose). Intolerance was defined as FEV1 decrease of 20% or greater, oxygen saturation less than 90% within 30 minutes post dose, severe coughing, chest tightness or dyspnea following nebulization.
3.3. Statistical Analysis
Statistical Package for Social Sciences (SPSS) 21.0 program was used for data analysis. Continuous and categorical variables were reported as mean ± standard deviation, and numbers or percentages, respectively. The Mann-Whitney Rank Sum test was performed to compare the continuous data between the groups. The Wilcoxon test was used to compare the continuous data in each group between entry and end study. The Fisher’s chi-square tests were used for the assessment of descriptive variable and McNemar’s test for microbiological responses like colony type (phenotype) and some of demographic data (e.g. sex). P value less than 0.05 was considered to be statistically significant.
4. Results
Sixty patients were met the criteria and entered the study. Ten participants in the control group and five in the intervention arm did not refer for day +28 assessment and were lost to follow-up. Therefore, 45 patients completed the trial, with 25 patients in the intervention arm and 20 patients in the control arm.
Baseline demographic characteristics of patients are outlined in Table 1. There were no statistically significant differences between treatment arms in demographic characteristics at baseline. None of the participants were receiving dornase alfa.
| Characteristic | Nebulized Azithromycin (N = 25) | Oral Azithromycin (N = 20) | P Value |
|---|---|---|---|
| Gender | 0.89 | ||
| Male | 12 (48) | 10 (50) | |
| Female | 13 (52) | 10 (50) | |
| Age, y | 12.92 ± 3.74 | 12.33 ± 3.62 | 0.58 |
| Weight | 35.2 ± 13.04 | 33.3 ± 10.8 | 0.64 |
| BMI | 16.44 ± 2.8 | 15.81 ± 1.9 | 0.62 |
| CFTR genotype | 0.91 | ||
| Homozygous for ΔF508 | 3 (20) | 3 (15) | |
| 9 (36) | 7 (35) | ||
| 12 (48) | 10 (50) | ||
| Other medications | |||
| Salbutamol | 22 (88) | 17 (85) | > 0.99 |
| Hypertonic saline | 22 (88) | 17 (85) | > 0.99 |
aValues are expressed as mean ± SD or No. (%).
4.1. FEV1 Improvement
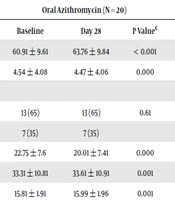
After 28 days of treatment, mean FEV1 percent change increased significantly from baseline in both treatment arms. However, participants receiving nebulized azithromycin had statistically significantly greater FEV1 % predicted improvement compared to oral azithromycin group (Table 2). Mean (95% CI) difference value of FEV1 in oral azithromycin group and nebulized group was 2.89 (3.34 - 2.44) and 8.78 (13.13 - 4.43), respectively.
| Measure | Oral Azithromycin (N = 20) | Nebulized Azithromycin (N = 25) | Treatment Effectb | |||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Day 28 | P Valuec | Baseline | Day 28 | P Value> | P Valued | ||
| FEV1% predicted | 60.91 ± 9.61 | 63.76 ± 9.84 | < 0.001 | 57.58 ± 12.82 | 66.36 ± 15.31 | 0.000 | 5.89 | < 0.001 |
| PA counte | 4.54 ± 4.08 | 4.47 ± 4.06 | 0.000 | 4.51 ± 4.37 | 4.37 ± 4.34 | < 0.001 | -0.5 | 0.005 |
| PA phenotype | ||||||||
| Mucoid | 13 (65) | 13 (65) | 0.61 | 18 (72) | 13 (52) | 0.61 | - | - |
| Non-Mucoid | 7 (35) | 7 (35) | 7 (28) | 6 (24) | ||||
| PedsQL-4 | 22.75 ± 7.6 | 20.01 ± 7.41 | 0.000 | 19.12 ± 12.78 | 11.28 ± 10.43 | 0.000 | -5 | < 0.001 |
| Weight | 33.31 ± 10.81 | 33.61 ± 10.91 | 0.001 | 35.21 ± 13.41 | 36.01 ± 13.11 | 0.000 | 0.56 | 0.001 |
| BMI | 15.81 ± 1.91 | 15.99 ± 1.96 | 0.001 | 16.44 ± 2.81 | 16.85 ± 2.88 | 0.000 | 0.23 | 0.005 |
Abbreviation: PA, Pseudomonas aeruginosa.
aValues are expressed as mean ± SD or No. (%).
bThe treatment effect was defined as the difference between the mean changes in the nebulized and oral azithromycin groups
cP values for the comparison of mean scores between baseline and day 28, by using paired-sample t-test.
dP values for the comparison of mean difference between oral azithromycin and nebulized azithromycin groups, by using univariate analysis of variance adjusted for baseline values.
eCount is reported as log10 PA CFU/g sputum.
4.2. Pseudomonas aeruginosa Colonization Characteristics
Neither oral azithromycin nor nebulized azithromycin affected the mucoid and nonmucoid phenotype of PA. However, sputum density of PA decreased significantly after oral and nebulized azithromycin. The magnitude of decline was greater in the patients who received nebulized azithromycin (Table 2).
4.3. Quality of Life
Quality of life as measured by PedsQL-4 questionnaire improved in both treatment arms. However, improvement was statistically significant in the nebulized group compared with oral azithromycin group (Table 2). Patient reported changes in quality of life included satisfaction from less frequent cough, decreased sputum viscosity and volume and change of its color from green/yellow to pale/white, as well as better school performance because of less leaving the classroom to clear the throat. In addition parents reported behavioral improvements of the children like being less stubborn and showing more happiness.
4.4. Weight and BMI
As illustrated in Table 2, after 28 days of treatment with azithromycin, patients in both treatment arms had statistically significantly increased weight and BMI, patients receiving nebulized azithromycin had statistically significantly greater improvement compared with children receiving oral azithromycin.
4.5. Adverse Effects
There were no cases of intolerance following the first dose (test dose) of azithromycin nebulizer administered at CF clinic. Thereafter, based on patients’ reports, productive cough was the only complaint more reported in the nebulized group in the first week which declined thereafter. Diarrhea, abdominal cramp and heart burn were statistically significantly more reported in the oral azithromycin arm.
5. Discussion
To the authors’ knowledge, this is the first study evaluating the efficacy of nebulized azithromycin in children with CF. The results of this study suggest that nebulized azithromycin may provide a new therapy for children with CF who have moderate to severe lung disease which is well tolerated. In addition, better improvements in pulmonary function, PA count, quality of life, weight and BMI were evident for nebulized compared with oral azithromycin. Whether this beneficial effects observed, last longer than 28 days, remains to be elucidated.
Several therapies are recommended for CF lung disease as mentioned in guidelines (14). Chronic use of inhaled tobramycin, dornase alfa, inhaled hypertonic saline and oral azithromycin are recommended by CF foundation to improve lung function and reduce exacerbations (14). These therapies generally result in a 3% - 5% improvement in FEV1 (23). The improvements of FEV1 observed in patients receiving oral azithromycin (2.89%) in our study is consistent with this. However we observed a much greater improvement in FEV1 in the nebulized azithromycin group (8.78%), which might be explained by potential superior route of delivery of anti-inflammatory therapy. This needs to be evaluated in further studies.
Nebulized and oral route deliver antibiotic to conductive and respiratory zones (9). Since, PA colonizes both zones (9), combined systemic and nebulized azithromycin is suggested as a new therapeutic strategy to intensify treatment of this life-limiting disease.
In the present study, sputum density of P. aeruginosa decreased, however, azithromycin did not affect the mucoid phenotype in both treatment groups. These could be interpreted as anti PA activity of azithromycin attributed to its antivirulence properties (8). Although not affecting mucoid phenotype, azithromycin reduced PA count, which could be explained by other aspects, like quorum sensing systems and pleiotropic properties on PA (8).
In this study, quality of life improved in both treatment groups, however, the magnitude was greater in nebulized group. Importantly patients’ self-reported outcomes were only announced with nebulized azithromycin. It has been suggested that CF patients reliably report their symptoms. Reliability of patient-reported symptoms are supported by clinical trials and US Food and Drug Administration (FDA) (24, 25).
In our study, improved weight gain was noted with nebulized azithromycin. Weight gain associated with azithromycin has been reported (26, 27). Mayer-Hamblett et al. evaluated the effects of addition of oral azithromycin to inhaled tobramycin as eradication regimen of new PA (26). The authors observed decreased pulmonary exacerbations and increased weight in the azithromycin group. Weight increased by an average of 1.27 kg with azithromycin during 18 months follow-up. However, here we report ameliorated weight gain with nebulized azithromycin.
The main limitation of this study was the short term follow-up to assess endpoints of time to next exacerbation and frequency of exacerbations. In addition we had a small number of participants. Also measurement of more sensitive endpoints of lung function like lung clearance index by multiple breath washout as recommended recently (23), was not assessed. Moreover, pharmacokinetics and pharmacodynamics of the intravenous formulation of azithromycin which was used as nebulized route in this study were not known. Appraising efficacy of this modality with a commercially available formulation is mandatory. Finally, the correct dose of nebulized azithromycin needs to be determined.
5.1. Conclusions
Results of this preliminary study showed that inhaled azithromycin may have a role in improving the patientcare of CF sufferers. Whether it could replace or be used in addition to oral azithromycin needs further research. Likewise, further studies with longer duration of follow-up are needed to elucidate its role in repeated courses in combination with inhaled antibiotics in long term treatment of CF lung disease. Further clinical trials with novel nebulizer formulation of azithromycin and large number of participants are needed to further assess the efficacy, safety and sustained effect of this new therapeutic approach in children with CF.