1. Background
Neonatal Jaundice is the most common neonatal illness and is the most common cause of hospitalization (1). In about 8 to 11 percent of cases, bilirubin levels elevate up to 95th percentile of the normal range and need to be followed and treated. In the absence of proper treatment, dangerous complications such as kernicterus can cause life-long disability (2, 3). Care, support, and lactation training before and after birth are important to prevent pathologic and life-threatening jaundice (4). Also, early detection and appropriate treatment reduce complications of jaundice (5). Therapeutic interventions in hyperbilirubinemia newborns include phototherapy and blood exchange transfusion (6).
Currently, the most effective and commonly used treatment for neonatal jaundice is phototherapy, which is a safe treatment in term and preterm infants (5, 7). Phototherapy reduces the risk of blood exchange by decreasing total bilirubin concentration (8), but there are rare reports of significant toxicity in neonates. Generally, the complications of phototherapy can be categorized as follows: (A) Short-term complications include: separation between mother and baby, ambient temperature imbalance, water loss, electrolyte disorders (especially hypocalcemia), conjunctivitis, sleep disturbances, tanning child syndrome; (B) long-term complications include the likelihood of an allergic disease (such as allergic rhinitis and asthma), melanocytic macular degeneration, melanoma, and skin cancers, open ductus arteriosus (PDA) and retinal damage (9).
It is reported that newborns with jaundice requiring phototherapy are at increased risk of developing asthma at a later stage of life than other infants who do not experience jaundice (10-12). During phototherapy, erythema rash may appear as a side effect in a temporary way (13). Eosinophils are differentiated bone marrow-derived granulocytes that play an essential role in the pathogenesis of asthma (14). The vascular cell adhesion molecule (VCAM-1) cellular connection molecule plays a very important role in the migration of inflammatory cells in the endothelium, which causes the binding of inflammatory cells, such as eosinophils and lymphocytes, to vascular endothelial cells (15). Bilirubin has been shown to inhibit the connection of the VCV-1 cell line (VCAM-1) with an undefined mechanism, also decreases in interleukin 2 and 10 levels resulting in increased levels of eosinophils and lymphocytes in the pulmonary ducts (16).
2. Objectives
So far, very limited studies have shown that phototherapy affects the level of eosinophils. Therefore, this study examined the relationship between phototherapy and bilirubin level on blood eosinophils.
3. Methods
In this prospective cross-sectional study, patients who were admitted for jaundice in neonatal or neonatal intensive care unit of the Children’s Medical Center Hospital, Tehran, Iran from 2017 to 2018 were studied. The indication of phototherapy was determined according to the phototherapy nomogram of the American Academy program (4).
Patients’ laboratory data, such as serum bilirubin and the number of blood cells, differential eosinophils count, before, during, and after receiving phototherapy were collected. Demographic data including sex, gestational age, and the age of the baby at the start of phototherapy, number of phototherapy days, also hemolytic and non-hemolytic causes of hyperbilirubinemia were recorded in a checklist designed according to the goals of the study.
CBC was performed by Coltter Counter method at baseline and one, two, and four days after phototherapy. The number of eosinophils in peripheral blood was evaluated using absolute eosinophils count. The number of eosinophils less than 400/μl considered normal, and eosinophils more than 700/µL as eosinophilia [17,18].
Exclusion criteria were: (1) the presence of any underlying illness; (2) discontinuation of phototherapy before 24 hours; (3) consuming any medication other than vitamin drops; (4) eosinophilia at the beginning of hospitalization; (5) congenital major anomalies; (6) need to exchange transfusion before one day of phototherapy; (7) lack of sequential CBC check-up during phototherapy; and (8) lack of blood sampling permission by the baby’s parents.
This study was designed and performed based on the ethical principles of the Helsinki declaration, and approved by the Ethics Committee of Tehran University of Medical Sciences (ID: 1396.3706). A full explanation was given to parents about the study and written informed consent obtained from them.
3.1. Sample Size
The G*Power 3 software was used to calculate the sample size. Error probability coefficient α (0 ≤ 0.05) and Power (1-β err prob.) = 80% were used and the result (based on the type of analysis) was used to determine the required sample size. Considering the mean and SD of eosinophils count before and after phototherapy, according to previous studies (17), we achieved a minimum sample size of 144 patients.
4. Results
A total of 163 newborns meeting inclusion criteria were evaluated. The mean age of newborns was 5.49 ± 4.01 days (range 1-30 days), and the mean serum total bilirubin concentration in the newborns was 17.91 ± 3.37 mg/dL (range 8.3-25.6 mg/dL). The descriptive demographic characteristics of newborns are listed in Table 1.
| Variable | Values |
|---|---|
| Sex | |
| Male | 85 (52.1) |
| Female | 78 (47.9) |
| Age, wk | |
| < 1 | 134 (82.2) |
| 1 - 2 | 23 (14.1) |
| > 2 | 6 (3.7) |
| Maturity | |
| Preterm | 31 (19) |
| Term | 132 (81) |
| Jaundice type | |
| Hemolytic | 23 (14.1) |
| Non-hemolytic | 140 (85.9) |
aValues are expressed as No. (%).
According to Kolmogorov Simonov test, the numerical values of the mean eosinophil count before phototherapy up to 4 days after its start did not follow a normal distribution (P < 0.05). The mean values of eosinophils before phototherapy and one/two/four days after starting this treatment are shown in Table 2.
| Variable | Number of Evaluated Patients | Values | Min-Max |
|---|---|---|---|
| Before PT | 163 | 377.96 ± 181.24 | 0 - 799 |
| The first day after PT | 163 | 467.12 ± 323.96 | 0 - 1840 |
| The second day after PT | 58 | 640.24 ± 339.97 | 0 - 1491 |
| The fourth day after PT | 1 | 1420 | - |
aValues are expressed as mean ± SD.
Compared to the mean value of eosinophils before phototherapy, this value in the first (P = 0.001) and second (P < 0.001) days were significantly higher. Also, eosinophil count was calculated for one patient on the fourth day, which showed a significant increase compared to the average of the previous days; however, this day was excluded from further analysis.
The mean values of eosinophils in the first and second days of phototherapy were compared with before treatment based on the gender of infants, age of phototherapy, presence/absence of hemolysis, and gestational age are presented in Table 3.
| Variable | Mean 1st PT Day Eos | Za | P Value | Mean 2nd PT Day Eos | Z | P Value |
|---|---|---|---|---|---|---|
| Sex | ||||||
| Male | 436.42 | -1.24 | 0.21 | 552.53 | -3.29 | < 0.01b |
| Female | 500.57 | -3.36 | 0.00b | 722.10 | -4.41 | < 0.01b |
| Age at PT, wk | ||||||
| < 1 | 473.82 | -2.94 | 0.00b | 591.36 | -4.56 | < 0.01b |
| 1 - 3 | 438.69 | -1.62 | 0.10 | 776.71 | -2.36 | 0.01b |
| > 2 | 426.33 | -0.10 | 0.91 | 975.75 | -1.82 | 0.06 |
| Hemolysis | ||||||
| Yes | 493.47 | -1.21 | 0.22 | 676.33 | -2.72 | < 0.01b |
| No | 462.79 | -3.10 | 0.00b | 627.65 | -4.70 | < 0.01b |
| Maturity at birth | ||||||
| Term | 476.70 | -4.08 | < 0.01b | 632.95 | -5.02 | < 0.01b |
| Preterm | 426.32 | -0.48 | 0.63 | 679.88 | -2.31 | 0.02b |
Abbreviation: Eos, eosinophils.
aWilcoxon test.
bP < 0.05.
In cases where the patient was a term neonate, the difference was significant between the eosinophils count before phototherapy and the first and second day. In the case of preterm infants, this difference before phototherapy and the first day after that was not significant, but an increase in the second day was significant.
At the beginning of the study, 17 patients who had eosinophilia were excluded. Subsequently, adding these cases to the study, we studied the effect of hyperbilirubinemia on eosinophilia after phototherapy. There was no significant correlation between total serum bilirubin levels in three regions less than 15, 15 - 20, and more than 20 mg/dL, and eosinophils count before and after phototherapy (P = 0.789). Regarding the abnormal distribution of the data, the Kruskal-Wallis test showed no significant association between the bilirubin groups with any of the eosinophils levels before phototherapy (P = 0.497), one day after phototherapy (P = 0.385) and two days after phototherapy (P = 0.976). This suggests that adding neonates with eosinophilia does not change the results.
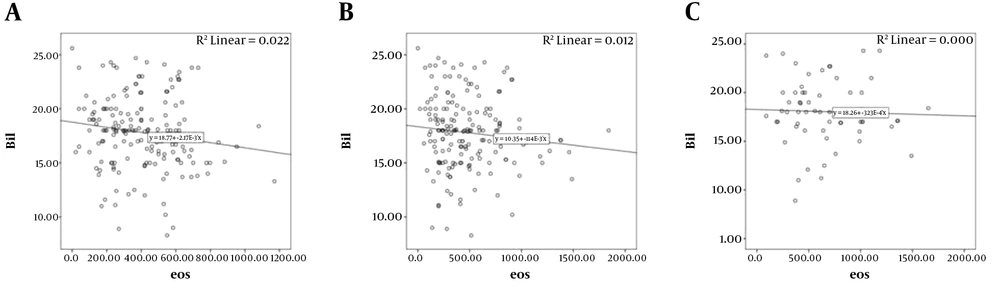
Using Spearman test, it was found that there is a significant negative correlation between the mean total bilirubin of patients with mean eosinophils before phototherapy (P = 0.019, correlation coefficient = -0.178), that is, with increasing bilirubin, the number of eosinophils are decreased, but there was no significant correlation between mean eosinophils count one day (P = 0.078, correlation coefficient = -0.134), and two days after phototherapy (P = 0.675, correlation coefficient = -0.055) (Figure 1).
5. Discussion
In the present study, we showed that the mean number of eosinophils before phototherapy was 377.96 ± 181.24, which significantly increased in the first and second days after phototherapy. This increase in eosinophils in male neonates observed in the second day and females in the first and second days after phototherapy. Also, in the newborns less than a week old, this difference was significant.
In line with our study, Ayden and colleagues showed that eosinophils count increased from f 402.27 to 506.4 after phototherapy. They showed that high levels of bilirubin may result in decreased levels of eosinophils and treatment of jaundice can increase the number of eosinophils (18).
The most common treatment for indirect hyperbilirubinemia is phototherapy. Recent studies have shown that the secretion of certain cytokines from peripheral blood mononuclear cells affected term neonates receiving phototherapy. Kondo and colleagues showed an increased level of IL-8 mRNA after exposing the normal skin to ultraviolet radiation (19). In another study, Sirota et al showed a decrease in the secretion of interleukin 1 beta and an increase in interleukin 2 and 10 after phototherapy (20). There is a hypothesis that eosinophilia can occur in correlation to phototherapy which can stimulate allergic diseases. On the other hand, bilirubin can be protective against allergic diseases. Some studies showed that infants who develop jaundice and receive phototherapy are 1.5 times prone to develop asthma (11) and one study showed that neonatal jaundice or receiving phototherapy can induce asthma after 12 years of age (10). But these studies do not indicate that asthma is associated with bilirubinemia or phototherapy.
Eosinophils are bone marrow-derived cells that ultimately differentiate into granulocytes involved in parasitic infections and allergic diseases (14). The measurement of its secreted derivatives, including ECP, is used to diagnose neonatal atopic dermatitis and bronchopulmonary dysplasia (21). Beken et al. (17) showed that although the level of eosinophils increases after phototherapy, this alteration was not significant, but the results indicated a significant increase in ECP eosinophilic derivatives. However, we showed a significant increase in eosinophils count especially with increasing days of phototherapy. The mean of eosinophils after 2 days of phototherapy was higher than the mean value of eosinophils in term neonates (22).
In female neonates, there was a significant increase in eosinophils in the first and second days of the treatment period. However, in male cases, despite the increase in eosinophils in the first day after phototherapy, no significant difference was observed. This suggests that female sex is associated with increased eosinophils after phototherapy, which may be due to the hormonal effects of this sex.
Our findings indicate that there is a significant relationship between hyperbilirubinemia and phototherapy and the increased level of peripheral blood eosinophilia, and this relationship is related to the age of the baby. This suggests that at an age of less than a week, the influence of exposure to phototherapy is higher than that of older ones, which can be due to the lack of maturity of the body’s early immune system at the beginning of life.
On the other hand, increased eosinophil levels in neonates with hemolytic jaundice was more marked than those with non-hemolytic type. Also the increase in the number of eosinophils in preterm infants in the second day after phototherapy was significantly higher in comparison with term neonates, which means that preterm infants are more likely to develop allergies or asthma in the future.
There was a significant negative correlation between the mean total bilirubin concentration of patients before phototherapy with mean eosinophilia, that is, with the increase of bilirubin, the number of eosinophils was decreased; however, the mean eosinophils count in the first and second days after phototherapy was not significantly correlated with the severity of hyperbilirubinemia.
5.1. Conclusions
Considering that in this study, the increased levels of bilirubin was associated with a reduction in the number of eosinophils, also, after phototherapy, the number of eosinophils increased with the duration of phototherapy, inflammatory reactions including asthma in icteric children can be attributed to phototherapy. Therefore, with regard to complications of phototherapy, long-term and unnecessary phototherapy can be avoided. Of course, further studies and follow-up of eosinophilic neonates are needed to come to a more comprehensive conclusion.