1. Background
Celiac disease (CD) is an immune-mediated systemic disease triggered by gluten intake in genetically susceptible individuals and affects approximately 1% of the general population. The clinical findings of celiac disease change over time. Instead of classical symptoms, atypical symptoms such as diarrhea, growth retardation, abdominal distention and chronic constipation may be the only sign of celiac disease, and also no symptoms may be seen (1).
Type 1 diabetes mellitus (DM) is an autoimmune disease characterized by insulin deficiency as a result of autoimmune destruction of beta cells (2).
Type 1 DM, autoimmune disorders, relatives of celiac patients have higher risk of developing CD because they share the same HLA type (3). Celiac disease and type 1 DM are autoimmune and common in children. Both diseases have a common genetic locus on the short arm of chromosome 6. Because they have a common genetic background, both diseases frequently occur (4). Also, different non-HLA locations associated with CD and type 1 DM has been reported (5).
The relationship between type 1 DM and CD is well known, and the prevalence of biopsy-proven CD has been reported to be 1.6% - 16.4% (6-10).
Timely diagnosis of celiac patients is important in preventing long-term complications such as osteopenia, growth retardation, infertility and malignancies associated with untreated disease (11, 12).
Early diagnosis of CD in patients with type 1 DM has been reported to improve patients’ clinical parameters such as weight and serum ferritin levels, and have positive impact on quality of life (13, 14).
2. Objectives
According to international guidelines, serological screening for CD in children and adults with type 1 DM is recommended but there is no consensus on how often it will be performed.
In the present study, we aimed to investigate the prevalence of CD in children with type 1 DM.
3. Methods
This present study was carried out between 01 March 2017 and 15 December 2018 in the Clinics of Pediatric Gastroenterology and Endocrinology. 273 children with type 1 DM were included in the study. Patients with concomitant autoimmune diseases and patients who refused to participate were excluded from the study.
One patient diagnosed with CD before the diagnosis of type 1 DM was excluded from the study. Written informed consent was not obtained as the study is retrospective. But The Local Ethics Committee approved the current study (10 January 2019-2019/08).
We used the ESPGHAN guideline of celiac disease “Asymptomatic Child or Adolescent With CD-associated Conditions”. If HLA testing is available, it should be offered as the first line test. If HLA testing is not done, then an anti-TG2 IgA test and total IgA determination as first line tests should be performed. If antibodies are negative, then repeated testing for CD-specific antibodies is recommended. To avoid unnecessary biopsies in individuals with low-CD specific antibody levels (i.e. < 3 times ULN), it is recommended that the more specific test for anti-endomysial antibody (EMA) be performed. If the EMA test is positive, then the child should be referred for duodenal biopsies. If the EMA test is negative, then repeated serological testing on a normal gluten-containing in diet 3 to 6 monthly intervals is recommended (1).
Patients were evaluated in terms of clinical and laboratory findings of CD. Tissue transglutaminase antibody IgA (tTG IgA) and total IgA levels were measured in all patients (1). The normal range of tTG IgA and EMA IgA is below 20 U/L. The cutt-off value of IgA level is 5 mg/dL. Tissue transglutaminase antibody IgG was analysed in patients with IgA deficiency (15).
The patients with tTG IgA positivity underwent gastroduodenoscopy. At least four biopsies from duodenum and one biopsies from bulb were obtained. All biopsies were evaluated according to the Marsh classification criteria (16). Marsh stage 0: normal mucosa, Marsh stage 1: increased intraepithelial lymphocytosis (> 40 lymphocytes per 100 epithelial cells), Marsh stage 2: increased intraepithelial lymphocytosis with crypt hyperplasia, Marsh stage 3a: increased intraepithelial lymphocytosis with crypt hyperplasia and partial villous atrophy, Marsh stage 3b: increased intraepithelial lymphocytosis with crypt hyperplasia and subtotal villous atrophy, and Marsh stage 3c: increased intraepithelial lymphocytosis with crypt hyperplasia and total villous atrophy.
Statistical analysis was performed using SPSS software version 13.0 (SPAA Inc, Chicago IL, USA). Categorical data were reported as percentages and continuous data as mean standard deviation (SD) or median (interquartile range). Independent-Samples t-test was used for nominal data with normal distribution. Mann Whitney U test was used for the parameters which did not show a normal distribution.
4. Results
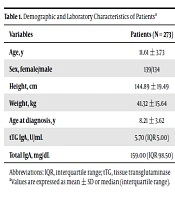
Of the 273 patients (139 girls), the mean age was 11.61 ± 3.73 years and the mean age of patients at the diagnosis of type 1 DM was 8.21 ± 3.62 years (Table 1). Tissue transglutaminase antibody IgG was analysed in three of patients who have IgA deficiency, and no positivity was found.
In our study, tTG IgA was positive in 23 patients, and 2 of them who have no symptoms of CD refused the process of endoscopy. 6 patients had low tTG levels (< 3 times ULN), EMA test is analysed in those patients to avoid unnecessary biopsies. All of them had EMA positivity. Thus, gastroduodenoscopy was performed on other 21 patients. 11 patients with Marsh stage 3, 2 patients with Marsh stage 2, 4 patients with Marsh stage 1, and 4 patients with Marsh stage 0 were detected in the present study. In other words, 12 patients were diagnosed with CD, 5 patients were diagnosed with potential CD (Table 2). Those patients were followed-up. One of the patients with Marsh stage 2 was found to have helicobacter positivity. After the eradication treatment of Helicobacter pylori, endoscopy was performed, and multipl biopsies were obtained from the duodenum and bulb. The result of pathology was consistent with Marsh stage 0.
| Variables | Patients (N = 273) |
|---|---|
| Age, y | 11.61 ± 3.73 |
| Sex, female/male | 139/134 |
| Height, cm | 144.89 ± 19.49 |
| Weight, kg | 41.32 ± 15.64 |
| Age at diagnosis, y | 8.21 ± 3.62 |
| tTG IgA, U/mL | 5.70 (IQR 5.00) |
| Total IgA, mg/dL | 159.00 (IQR 98.50) |
Abbreviations: IQR, interquartile range; tTG, tissue transglutaminase
aValues are expressed as mean ± SD or median (interquartile range).
| Patient No. | Age, y/Sex | Age at Diagnosis of DM, y | tTG IgA, U/mL | Pathology |
|---|---|---|---|---|
| 1 | 8.8/fa | 5.0 | 126.0 | Marsh 3a |
| 2 | 8.8/fa | 3.5 | 195.0 | Marsh 3b |
| 3 | 13.2/ma | 3.0 | 181.0 | Marsh 3a |
| 4 | 11.3/fa | 9.7 | 142.0 | Marsh 3a |
| 5 | 4.0/ma | 1.7 | 124.4 | Marsh 3a |
| 6 | 10.0/fa | 5.8 | 186.2 | Marsh 3a |
| 7 | 6.5/ma | 1.5 | 135.0 | Marsh 3c |
| 8 | 13.5/fa | 12.5 | 142.0 | Marsh 3a |
| 9 | 13.5/ma | 5.0 | 172.0 | Marsh 3a |
| 10 | 5.5/ma | 5.0 | 25.4 | Marsh 3a |
| 11 | 12.1/fa | 11.8 | 142.0 | Marsh 3a |
| 12 | 9.0/fa | 5.9 | 49.8 | Marsh 2 |
| 13 | 7.6/f | 4.1 | 69.8 | Marsh 2 |
| 14 | 6.8/f | 6.6 | 35.2 | Marsh 1 |
| 15 | 9.3/m | 2.0 | 34.1 | Marsh 1 |
| 16 | 17.0/f | 4.0 | 99.6 | Marsh 1 |
| 17 | 6.5/m | 3.0 | 168.3 | Marsh 1 |
| 18 | 10.0/m | 5.0 | 40.7 | Marsh 0 |
| 19 | 9.1/f | 8.5 | 166.0 | Marsh 0 |
| 20 | 10.5/m | 9.0 | 185.9 | Marsh 0 |
| 21 | 9.7/m | 9.5 | 42.5 | Marsh 0 |
Abbreviation: DM, diabetes mellitus
aAge at diagnosis of celiac disease
Seven of 12 celiac patients was asymptomatic. Three of the symptomatic patients had failure to thrive, one patient had chronic constipation, and one had anemia. The seroprevalence of CD and biopsy-proven prevalence of CD were 8.4 and 4.4%, respectively.
In our study, 9 of 12 patients diagnosed with CD were diagnosed within the first 5 years after the diagnosis of type 1 DM, four of them were diagnosed within 2 years.
5. Discussion
The prevalence of CD has increased dramatically in the last two decades due to the use of sensitive and specific serological tests and a better understanding of the disease by physicians (17). Although serological tests for CD are recommended in high-risk groups, the majority of asymptomatic patients are still undiagnosed (18).
In a systematic review and meta-analysis, the prevalence of biopsy-proven CD was reported to be 0.7% in the general population worldwide (19).
The prevalence of biopsy-proven CD in type 1 DM has been reported to be 1.6% - 16.4% (6-10). As consistent with the literature, the biopsy-proven prevalence of CD in children with type 1 DM was 4.4%.
ESPGHAN and BSGHAN recommend HLA DQ analysis in addition to tTG IgA test as the first-choice screening test in high-risk groups such as type 1 DM. It was suggested that no further examination is necessary for CD in patients with negative findings (1, 20).
In addition to that, it has been reported that HLA typing was not cost-effective in patients with type 1 DM, as HLA-DQ2 / 8 positivity was detected in approximately 90% of patients (21, 22). Due to the high cost, HLA typing is often not possible. In our study, we could not perform HLA typing because of the high cost.
It has been reported that approximately 85% of type 1 DM patients diagnosed with CD were asymptomatic in a systematic review and meta-analysis (23). As compatible with literature, 7 of 12 patients (58.3%) diagnosed with CD are asymtomatic.
In a systematic review, it has been recommended that screening tests for CD should be performed within the first 2 years and 5 years after the diagnosis of type 1 DM, since CD is usually diagnosed within 5 years in type 1 DM patients (23). As consistent with the literature, 9 of 12 patients diagnosed with CD were diagnosed within the first 5 years after the diagnosis of type 1 DM in our study. Also, four of those patients were diagnosed with CD within 2 years after the diagnosis of DM.
The risk of developing CD is higher in female gender and younger patients diagnosed with DM (6, 24, 25). As consistent with those studies, 7 of our patients diagnosed with CD were 5 years or younger, but there was male dominance. The reason for this gender difference may be a cross-sectional study.
In a multicenter study including healthy school age children, the prevalence of CD was found to be 0.47% in our country (26). In the curent study, the prevalence of biopsy-proven CD in children with type 1 DM was found to be 4.4%, which is approximately 9 times higher than in the general population.
It has been reported that patients with undiagnosed CD and DM had worse glycemic control and a higher prevalence of retinopathy and nephropathy (27, 28). In those patients, 1-year gluten-free diet was found to be safe and had no negative effect on quality of life.
5.1. Limitations
First, two of our patients refused the process of endoscopy because having no symptoms associated with CD. If he had accepted the gastroduodenoscopy, the biopsy-proven prevalence of CD would be higher. Second, HLA-DQ analysis could not be performed to patients due to the high costs of HLA analysis. Third, because CD can be diagnosed at all stages in life, the follow-up period may be short. Therefore, we may have detected a less prevalence of CD than expected.
5.2. Conclusions
We found that the prevalence of biopsy-proven CD in children with type 1 DM was 4.4%, which was approximately 9 times higher than the prevalence of CD in the general population. In the current study, 9 of 12 patients diagnosed with CD were diagnosed within the first 5 years after DM. In addition, 58.3% of our patients with CD were asymptomatic. According to our results, we recommend that screening tests for CD should be performed at least once a year for 5 years in children with Type 1 DM, even if the patients are asymptomatic.