1. Background
Jaundice can be observed in two-thirds of infants. In most cases, it is physiologic Jaundice and self-limited. However, one in five cases needs further investigation and about 10% of these patients will need treatment. Without proper treatment or delay in seeking medical attention, bilirubin accumulation may lead to life-long complications (1, 2).
Physiologic jaundice is a normal response to neonate’s limited ability to uptake bilirubin during the first few days after birth. Since long ago physiologic jaundice has been viewed as harmless, however, in recent decades a few benefits have been suggested such as antioxidant properties of bilirubin. Bilirubin is an oxygen-producing unit and acts similar to an antioxidant, especially while bonded to albumin (3). There are several debates on the mechanism and the effect of bilirubin on antioxidant system of the body. Bilirubin prevents DNA damage by oxidative stress of reactive oxygen species (ROS). Oxidative stress can be defined as an imbalance between amounts of ROS and extracellular antioxidant defense systems (4, 5). Moreover, bilirubin has anti-inflammatory and anti-apoptotic effects in addition to antioxidant properties (6). The antioxidant role of bilirubin has been demonstrated in several in-vitro studies. It is probable that in mice during the first few days after birth bilirubin of the serum protects them against oxidative damage (7). The comparison of antioxidant activity of bilirubin and ascorbate in neonatal plasma suggested that there’s a direct correlation between antioxidant activity of the plasma and bilirubin levels (8).
On the other hand, treatment of physiologic jaundice is not risk-free, therefore overtreatment must be done with caution (9). The most common and effective treatment for jaundice is phototherapy which is known to be a safe and tolerable method in infants (10). However, in recent decades phototherapy has been determined as an oxidative agent that can cause peroxidation of lipids and damage to the DNA (11-13). Traditional phototherapy has a negative effect on the oxidant-antioxidant defense system in infants born at term (13).
2. Objectives
Therefore, despite the benefits of phototherapy, it can cause complications due to a reduction in the infant’s antioxidant potency. Infants are in general more prone to oxidative stress and damage caused by free radicals. Furthermore, antioxidant defense mechanisms are not fully developed in infants and bilirubin can partially improve the complications caused by a lack of antioxidants. Considering these facts, we decided to compare pro-oxidant-antioxidant balance (PAB) in physiologic and pathologic jaundice.
3. Methods
This cross-sectional study included 171 term and near term neonates who were admitted to the clinic or the emergency room of Ghaem Hospital in Mashhad from 2017 to 2019. Based on the American Academy of Pediatrics (AAP) guidelines (14) we enrolled the neonates with more than 35 weeks of gestational and 2 days of postnatal age and categorized them into two groups of physiologic and pathologic jaundice. Consent forms were signed by parents before entering the infants into this study. The present study has been approved by the Ethics Committee of the Medical University of Mashhad.
During the first two weeks, Infants admitted to the Neonatal Clinic were inspected and examined for jaundice, which was measured using a jaundice meter. Infants with jaundice meter values lower or equal to 14 mg/dL were categorized as physiologic jaundice (control group); those with values higher than 15 mg/dL, if confirmed with laboratory assessment of the plasma bilirubin, were put in the pathologic or case group. Neonates with sepsis, respiratory distress, hypoxic-ischemic encephalopathy, congenital anomaly, Rh and ABO incompatibility, indirect hyperbilirubinemia or maternal gestational diabetes, eclampsia, and pre-eclampsia were excluded. Infants in the case group needed phototherapy in days 3 to 14 postnatal and were transferred to the pediatric emergency ward and neonatal intensive care unit (NICU). The records for infants in the case group contained age, gender, birth weight, gestational age, Apgar score and mother’s medical history including age, parity, mode of delivery, pregnancy complications, delivery complications, hospitalization or exchange transfusion in previous children and blood type. Bilirubin levels, hematocrit, direct and or indirect coombs, reticulocyte count, glucose 6 phosphate dehydrogenase (G6PD) and PAB were evaluated. The start of phototherapy was based on the principles of AAP. At least 0.2 cc serum was sent under cold chain conditions to Bu-Ali Research Institute, Mashad, for PAB evaluation. PAB evaluation in this center is done using the following method.
Antioxidant levels are evaluated with 3.3 - 5.5 tetramethyl benzidine (TMB) staining and oxidation of colored cations. The mix ratio (0% - 100%) of the standard solution is 250 µmol of hydrogen peroxide with 3 mmol of uric acid in 10 mmol of NaOH. The TMB cation is prepared by mixing 60 mg of TMB powder into 10 to 20 mL of the solution and placed in a dark dry container for 2 hours. Afterward, 25 units of peroxidase enzyme are added to 20 mL of the solution which is distributed in each milliliter of the solution and kept at 20°C. To prepare TMB, 200 mL of TMB is added to 10 mL of acetate (0.05 mol of a buffer, pH = 5.8) and then mixed with 1 mL of TMB cation and 1 mL of TMB solution. The final solution is kept in a dark dry container for 2 minutes. Then 10 µL of each sample is standardly mixed with 200 µL of working solution, placed in 96 plates and kept in the dark for 12 minutes at 37°C. Finally, 100 µL of 2 N (normal) hydrochloric acid (HCL) solution is added to each dish and evaluated with the enzyme-linked immunosorbent assay (ELISA) which uses the wavelengths of 450 and 620 nm. From these standard samples, a standard curve is produced which demonstrates PAB value per HK unit. The effect of hydrogen peroxide on the standard solution is shown on this curve. The value of the samples is calculated fundamentally and demonstrated on a curve. The PAB is a laboratory indicator of the oxidant-antioxidant state; so it must be contemplated that the increase in oxidative stress can elevate the pro-oxidant-antioxidant balance and reciprocally increase in antioxidant activity can decline this balance. After data collection, the data was entered into version 23 of SPSS. t-test, chi-square, and Kolmogorov-Smirnov were used in the analysis. We considered a P value less than 0.05 for statistical significance.
4. Results
The average age of the enrolled neonates was 7.3 ± 4.11 days and more than half of the considered infants were male, 52.4% male against 47.6% female. The type of delivery in 52.3% of them was c-section, and 47.7% were delivered vaginally. Among the 171 evaluated infants, 74 (43.3%) infants had physiologic jaundice and 97 (56.7%) infants had pathologic jaundice. The average level of the plasma bilirubin in the pathologic jaundice group was 20.89 ± 5.50 mg/dL. It should be noted that in the physiologic jaundice group we have measured jaundice only transcutaneously.
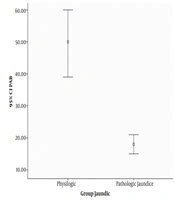
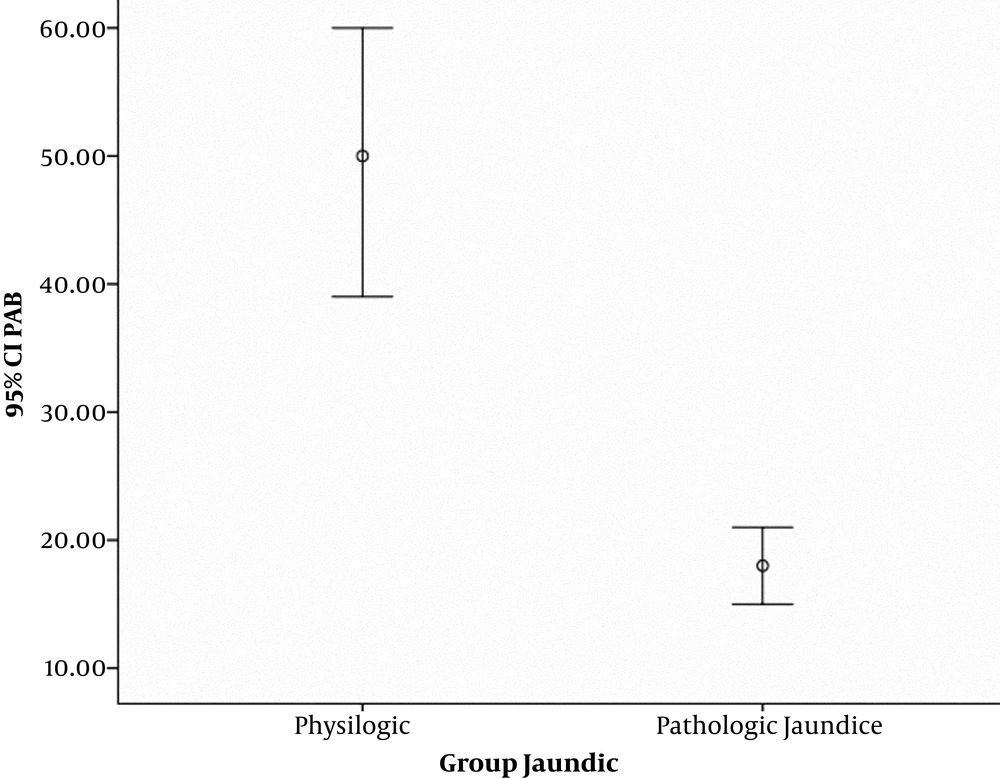
The mean values for neonatal and maternal parameters including neonate’s gender, gestational age, birth weight, Apgar score, mode of delivery, mother’s age and parity were compared between physiologic and pathologic jaundice groups. Statistically, there was a significant difference in gestational age (P = 0.010), parity (P = 0.001) and PAB (P = 0.000) between the two groups, meaning that the amount of these parameters were lower in the group of infants with pathologic jaundice than the group with physiologic jaundice. Figure 1 shows the difference of PAB parameter between the two groups. However, there was no significant difference between the two groups regarding the average birth weight, Apgar score and mother’s age. The obtained data are presented in Tables 1 and 2. Besides, the delivery mode between two groups of infants with physiologic and pathologic jaundice had a significant statistical difference (P = 0.039) but there was no significant difference regarding gender (P = 0.656) (Table 3)
5. Discussion
According to our study, pro-oxidant balance in infants with physiologic jaundice was more than twofold its level in pathological jaundice. As shown in Table 2, the median PAB level in infants with pathologic jaundice was 15.89 HK. In a study, PAB level in infants with jaundice who needed treatment was reported 16 HK (9). In another research, PAB level in infants with jaundice who needed blood exchange was reported 19.06 HK (12). The median PAB level in infants with physiologic jaundice was 38.27 HK. In other words, results of the present study showed that PAB levels are increased in the group of infants with physiologic jaundice in comparison with the group with pathologic jaundice which indicates bilirubin’s explicit role as an antioxidant. By reviewing available sources it is evident that the present study is one of the first researches which compare PAB levels in infants with physiological and pathological jaundice.
In recent years various articles have been published in this regard which can be summarized as follows: a study by Mayer considered bilirubin as a potential anti-oxidant factor, whose mild increase of its levels in the blood can be protective against some free radical related diseases (15). Additionally, Wiedemann et al. (16) showed a correlation between total levels of anti-oxidant and bilirubin levels in term and preterm neonates and disclosed that blood exchange decreases the concentration of bilirubin and thus the antioxidant capacity of the plasma. A study by Aycicek et al. (13) revealed the negative effects of phototherapy on oxidant/antioxidant system and an increase in oxidative stress levels in infants being treated with phototherapy. Furthermore, another study by the same author suggested that phototherapy can cause peroxidation of lipids and damage to DNA (11). In confirmation of the previous study, another research demonstrated that as an infant's bilirubin level decreases with phototherapy the balance of oxidant-antioxidant is disrupted toward antioxidant depletion (9). Results of the other investigation likewise showed that blood exchange causes an oxidant-antioxidant imbalance in favor of the oxidants (12).
Investigators in different studies tried to explain the bilirubin action mechanism as an antioxidant agent. Various mechanisms were proposed, such as the ability to excrete reactive oxygen species, inhibiting oxidation of LDL and chemotaxis of monocytes, improving endothelial function and an inverse association with changes of bisphenol A and phthalate levels in the urine (17-21).
It should be noted that regardless of the anti-oxidative role of the bilirubin, pathologic level of bilirubin has its formerly proven neurotoxicity. The reported mechanisms of destructive effects on the neurons of the brain are through decreasing oxygen consumption and increasing the release of calcium and caspase 3 resulting in apoptosis as well as a decrease of axonal and dendritic branching. It can also be effective in increasing apoptosis, disrupting oxidative stress and decreasing the synthesis of myelin in oligodendrocytes. Microglia reacts to the toxic damage of bilirubin by increasing the release of inflammatory precursors and the activity of metalloproteinases. Astrocytes demonstrate a pre-inflammatory pattern by increasing the release of glutamate resulting in apoptosis. Cells decrease the intracellular concentration of bilirubin through ATP-binding cassette (ABC) transporters or by increasing other less toxic metabolites using the bilirubin oxidase of p450 cytochrome or both mechanisms. This response protects the cell against other consequences of cellular damage. It has been recently determined that there is an inverse association between amounts of bilirubin in the brain and the expression of bilirubin metabolizing enzymes of p450. This suggests the probable role of these enzymes in the toxic placement of bilirubin in the cells and certain parts of the brain (22).
In a study by Dani et al. (23) a decrease in the plasma bilirubin was shown to have simultaneously increased the antioxidant capacity of the plasma and decreased the oxidative stress in preterm infants. The present work differs from the mentioned study in that we enrolled term and near term neonates, whereas they enrolled preterm neonates. The other is the assessment technique; we studied the global pro-oxidant-antioxidant balance, while they evaluated the components of the oxidative stress cycle. Dani et al. (24) in another research stated that the antioxidant effects may be observed only in the physiologic amount of the bilirubin, and that its pathologic levels are associated with pro-oxidant effects. Similar to the mentioned articles, another investigation performed by Rawat et al. (25) in neonatal mice declared that the potent antioxidant effect of the bilirubin was only observed at the moderate levels and it had pro-oxidant effects at high concentrations. Furthermore, in contrast to our results, they attributed the neurotoxic effects of the bilirubin to oxidative stress and other mechanisms (25). In the present study, pathological increase in bilirubin level, irrespective of its neurotoxic properties, activated diminishing mechanisms of oxidative stress and declined the pro-oxidant-antioxidant balance of the body. This means that even a pathologic increase of bilirubin leads to an increase of antioxidants levels, which can be detected in laboratory tests; it stays in contrast to the previous studies which reported antioxidant properties only for the physiologic amounts of the bilirubin (8, 24, 25).
In our study, pathological jaundice was more common in neonates born by normal delivery. Subsequent studies have reported inconsistent results between the delivery method and severity of hyperbilirubinemia (26, 27).
5.1. Conclusions
A pathologic increase in bilirubin level, irrespective of its neurotoxic effects, can decline oxidative stress and pro-oxidant-antioxidant balance. This means even high pathologic bilirubin concentrations can elevate antioxidants levels; however, it seems that toxic mechanism of bilirubin may be different from its antioxidant properties. More investigation is required to further understanding of the matter.