1. Background
Diarrheal diseases continue to be one of the main causes of morbidity and mortality worldwide. They pose a threat to all age groups, especially to children. It has been estimated that the incidence of diarrhea ranges from 0.5 to 2 episodes per child per year in children < 3 years in Europe (1). Diarrhea accounts for 9% of children’s death worldwide (2). Viral pathogens (norovirus, rotavirus) are more common in children < 5 years, bacterial agents (Clostridium difficile, E. coli, Salmonella spp., Campylobacter spp., Shigella spp.) in children > 5 years (3).
Neutrophils provide the first line of defense against invading pathogens and are the first cells recruited to the site of infection (4). Calprotectin (S100A8/A9) is a protein belonging to the S100 protein family. It comprises approximately 40% of total neutrophil protein contained in the cytosol (5). Activated neutrophils infiltrate intestinal mucosa and their secreted products can be detected in feces due to the release into the intestinal lumen (6). Activated and necrotic neutrophils are the main sources of extracellular calprotectin (7). Fecal calprotectin (FC) is a biomarker representing intestinal inflammation and its increase is proportional to the amount of neutrophils migrating to the intestinal lumen (8). A significant feature of bacteria-induced intestinal inflammation is a large influx of neutrophils into the intestinal tract mucosa (9). Experimental animal models demonstrate that infection with enteropathogenic viruses generally causes local, low grade intestinal inflammation with minimal cellular damage (10, 11). Those insights were applied in clinical practice and researchers present studies, demonstrating, that FC is a possible biomarker for differentiating bacterial and viral causes of infectious diarrhea (12). FC is believed to be a reliable marker in detecting intestinal inflammation. However, studies report that FC has age dependent distribution with higher concentrations detected in children than adults (13). This physiological increase of FC might be due to high permeability of the intestinal mucosa and increased neutrophil migration to the intestine (14).
2. Objectives
FC is widely used in clinical practice, however its suitability for pediatric patients is still under investigation. With this study we wanted to assess FC value as a biomarker in distinguishing viral and bacterial intestinal infection in different age groups of children.
3. Methods
3.1. Study Population and Material
This study was carried out in Children’s Hospital, it was performed from September 2016 to Otober 2018. A total of 143 children were invited to take part in the study. Study population comprised 103 participants with acute intestinal infections, hospitalized in Children’s Infectious Disease Department. According to etiological factor, test subjects were divided into two groups: viral pathogen group and bacterial pathogen group. Seventeen healthy control subjects, who had no current inflammatory disease comprised the control group. Venous blood and stool samples were obtained from test and control subjects. Twenty three participants provided insufficient samples or had multiple intestinal pathogens and were excluded from the study. Participant’s parents or legal guardians provided their agreement for participation in the study by signing a written informed consent form. Ethical approval for the research study was obtained.
3.2. Stool and Blood Testing
Etiological factors for intestinal infections were established by testing stool samples for pathogens. Each child provided two fecal samples. Samples without additives were divided into two parts: one part was used for immediate viral pathogen detection; the other was frozen at -80°C and further used for fecal calprotectin measurements. The other fecal sample came in Amies transport medium. Complete blood count was obtained from venous blood using an automated hematology analyzer (Sysmex XT 4000i, Roche, Germany). C reactive protein was measured in venous blood with cobas Integra 400 analyzer (Roche Diagnostics, Germany).
3.2.1. Virus Detection
Rota-, noro- and adeno-viruses were detected immediately using immunochromato-graphic stool test (Immunoquick, NoRotAdeno, Biosynex, Alsace, France). The test procedure was performed according to manufacturer’s instructions: 50 mg of solid or 50 µL of liquid feces were diluted with 800 µL of extract buffer, the sample was vortexed thoroughly, left to sit at room temperature for 5 min and centrifuged (3000 × g, 5 min), 90 µL of the obtained supernatant was added to the test cassette. After 15 min the appeared test and control indicative lines were evaluated.
3.2.2. Bacterial Detection
For microbiological examination stool samples were collected in Amies transport medium. Bacterial pathogens were identified by culturing methods. Stool cultures were inoculated on various selective and differential media (MacConkey agar, selenite broth, xylose lysine deoxycholate (XLD) agar, CIN), according to standard bacteriological procedures. Suspicious bacterial colonies were further isolated and differentiated using routine techniques, according to the suspected pathogen.
3.2.3. Fecal Calprotectin Measurement
Samples for FC concentration detection were kept frozen at -80°C. Stool samples were completely defrosted prior to testing. Ready to use stool extraction kit tubes were used for the extraction (EliATM, Fecal extraction device, Thermo Fisher Scientific, USA). For a liquid stool, 100 mg of a sample was weighed and diluted with 5 mL of extraction buffer (EliATM Calprotectin extraction buffer, Thermo Fisher Scientific, USA). Stool samples were completely homogenized with vortex, left to sit for 10 minutes at room temperature, then centrifuged (3000 × g, 5 min). Obtained supernatant was transferred to a new tube and used for further testing. Fecal calprotectin analysis was performed with fluorescence enzyme immunoassay, using Phadia Immunocap 100 analyzer (Phadia, Uppsala, Sweden). According to manufacturer, FC measuring range is: 0 - ≥ 3000.0 mg/kg. Samples with higher concentrations were diluted with extraction buffer (EliATM Calprotectin extraction buffer, Thermo Fisher Scientific, USA) and rerun.
3.3. Statistical Analysis
MS Office Excel, MedCalc software was used for data management and statistical analysis. Nonparametric data were presented with median and range. Mann-Whitney U test was used for comparing two groups of variables. Categorical data were expressed by a number and a percentage, chi-Square test was used to determine the significance of the difference. The most appropriate cut-off values for having viral and bacterial acute intestinal infections were determined by the area under the curve (AUC) using receiver operating characteristic (ROC) analysis. For each statistical test, that was used, P < 0.05 value was considered as significant.
4. Results
Viral pathogen test group comprised 72 children. Median age in this group was 2,0 years, ranging from 1 month to 6,7 years, 43% (n = 31) were males, 57% (n = 41) females. 56% (n = 40) of cases in viral pathogen group stool were positive for rotavirus, 37% (n = 27) norovirus, 7% (n = 5) adenovirus. Thirty one test subjects were included in the bacterial pathogen group. Age median in this group was 1.8 years, ranging from 3 months to 6.8 years. 58% (n = 18) were males, 42% (n = 13) females. 42% (n = 13) of bacterial pathogen group had Salmonella infection, 29% (n = 9) Campylobacter jejuni, 13% (n = 4) E. coli, 10% (n = 3) Yersinia enterocolitica, n = 1 Klebsiella pneumoniae, n = 1 Clostridium difficile. Control group comprised of 17 subjects; age ranged from 4 months to 6.7 years. 71% (n = 12) were male, 29% (n = 5) female. Detailed information is provided in Table 1.
| Characteristics | Viral Pathogen Group (N = 72) | Bacterial Pathogen Group (N = 31) | Control Group (N = 17) |
|---|---|---|---|
| Age, y | 2.0 (1.0 mo - 6.7 y) | 1.8 (3.0 mo - 6.8 y) | 2.3 (4.0 mo - 6.7 y) |
| Sex | |||
| Male | 31 (43) | 18 (58) | 12 (71) |
| Female | 41(7) | 13 (42) | 5 (29) |
| Identified pathogens | Rotavirus 40 (56) | Salmonella spp 13 (42) | - |
| Norovirus 27 (37) | Campylobacter jejuni (29) | ||
| Adenovirus 5 (7) | E. coli (13) | ||
| Yersinia enterocolitica 3 (10) | |||
| Klebsiella pneumoniae n = 1 | |||
| Clostridium difficile n = 1 | |||
| WBC × 109/L | 11.16 (3.78 - 29.31) | 10.59 (5.51 – 27.15) | 8.18 (5.6 - 16.59) |
| ANC × cells/µL | 8.32 (1.28 - 27.03) | 6.67 (1.41 - 23.39) | 2.41 (1.51 - 6.87) |
| CRP, mg/L | 7.0 (0.05 - 63.0) | 38.72 (0.76 - 118.0) | - |
Abbreviations: ANC, absolute neutrophil count; CRP, C-reactive protein; WBC, white blood cell count.
aValues are expressed as No. (%) or median (range)
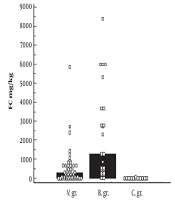
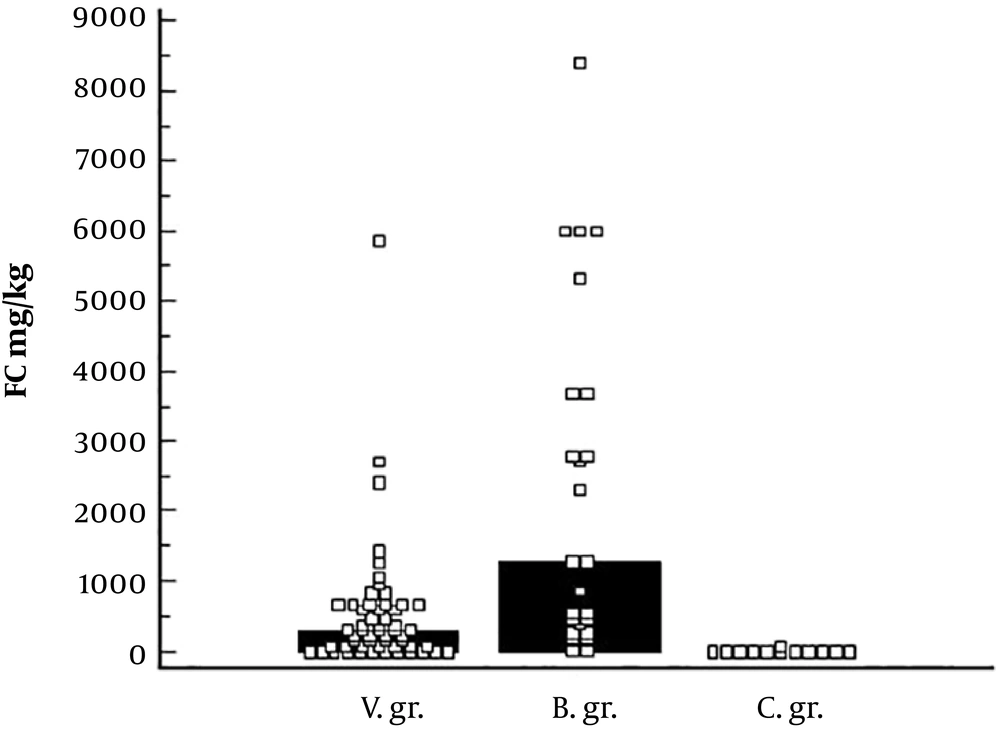
Fecal calprotectin values were higher in viral and bacterial pathogen groups, compared to control group: 297.0 mg/kg vs 6.45 mg/kg, P < 0.001; 1299.0 mg/kg vs 6.45 mg/kg, P < 0.001, respectively. Fecal calprotectin result was greater in bacterial pathogen group, compared to viral pathogen group: 1299.0 mg/kg vs 297.0 mg/kg, P = 0.002. See Figure 1.
The cut-off value of > 70.0 mg/kg (AUC = 0.942, P < 0.0001) for viral intestinal infection, with 78.2% sensitivity and 100% specificity, was determined. For bacterial intestinal infection: > 201.0 mg/kg (AUC = 0.983, P < 0.0001) with 88% sensitivity and 100% specificity. There was no difference in FC values between viral and bacterial pathogen group in children less than 1-year-old: 399.5 mg/kg vs 391.0 mg/kg, P = 0.945. FC concentration was significantly higher in bacterial pathogen group, compared with groups from 1 to 3 years: 316.0 mg/kg vs 1799.75 mg/kg, P = 0.001 and from 3 to 7 years: 99.0 mg/kg vs 1299.0 mg/kg, P = 0.044. See Table 2.
| Age Group | < 1 y | 1 - 3 y | ≥ 3 y |
|---|---|---|---|
| Viral pathogen, g | 9.0 mo (1 mo - 11 mo) | 1.8 y (1 y - 2.9 y) | 5.3 y (3 y - 6.7 y) |
| Median FC, mg/kg | 399.5 (64.0 - 1295.0) | 316.0 (12.0 - 1057.0) | 99.0 (0 - 5842.0) |
| Median CRP, mg/L | 7.0 (0.97 - 50.99) | 9.0 (0.05 - 159.0) | 3.01 (1.2 - 63.0) |
| Bacterial pathogen g, | 7.0 mo (3 mo - 8 mo) | 1.7 y (1 y - 2.6 y) | 6 (3.5 y - 6.8 y) |
| Median FC, mg/kg | 391.0 (71.0 - 3740.0) | 1799.75 (15.0 - 8412.0) | 1299.0 (273.0 - 5982.0) |
| Median CRP, mg/L | 42.5 (5.12 - 118.0) | 38.17 (0.76 - 118.0) | 38.72 (21.22 - 89.0) |
aValues are expressed as median (range).
5. Discussion
Quick discrimination between bacterial and viral etiological factors during acute intestinal infection is important for proper management of the disease (1). Microbiological stool testing takes long time and can be inconclusive due to false negative results, or presence of multiple pathogens (15). FC is used in clinical practice as a biomarker representing intestinal inflammation and its severity. Increase in FC concentration can be detected in the early phase of infection (16). In our study patients with acute intestinal infection had significantly higher FC values, compared to healthy controls. Overall bacterial pathogen group presented with higher FC concentration compared to viral pathogen group: 1299.0 mg/kg vs 297.0 mg/kg, P = 0.002. Chen et al. (12) study shows that FC was elevated in children with bacterial infectious diarrhea (12). Angela Lam et al. (17) report a significant positive correlation between the presence of intestinal pathogens and the increase of FC values in children up to 5 years, hospitalized with acute diarrhea. Asymptomatic infants and children with any enteropathogen had greater FC values compared to the group with no enteropathogens. The highest increase in FC level was reported in the group with multiple enteropathogens (18). According to our data children under a year old had similar FC values between bacterial and viral pathogen groups: 391.0 mg/kg vs 399.5 mg/kg, P = 0.945. The difference between bacterial and viral pathogen groups was significant in children from 1 year of age. Recent studies demonstrate that FC values are highly dispersed with higher concentrations detected in neonates and infants (13). Researchers report different FC values in healthy children. According to recent studies FC value of ≤ 50.0 mg/kg is acceptable for healthy adults and children over 4 years old (19). Researchers report slightly higher reference values in children from 1 to 4 years old (20). However, the biggest variability is seen in suggested reference values for children from birth to 1 year old. Hestvik et al. declare 249 mg/kg, but there are reports of suggested values up to three times higher (21, 22). The possible reason for this variability is that external factors influence FC test results. Asgarshirazi et al. (23) report that FC was significantly higher in exclusively breastfed infants, compared to formula and mixed fed infants. Lasson et al. (24) report that FC demonstrated a significant variability, testing stool samples collected during a single day. We hypothesize that higher nominal values and high variability could be the reason of being no difference in FC concentration in bacterial and viral pathogen groups in children under one year old. FC is a sensitive biomarker, representing intestinal inflammation; however, in children under one year old, alternative biomarkers, or a combination of several, are recommended (25, 26).
This study has several limitations. Firstly, limited number of test subjects, especially children under 1-year-old with acute bacterial intestinal infections. Secondly, the control group was small due to insufficient samples, with just a few healthy controls under 1-year-old. Finally, test and control subjects provided a single stool sample. There was no possibility to evaluate the possible variability.
5.1. Conclusions
This study demonstrates that a greater increase in fecal calprotectin concentration is associated with acute bacterial intestinal infections in children from 1-year-old. Children under 1-year-old showed no difference in FC concentration, between bacterial and viral acute intestinal infections. Further testing is needed.