1. Background
Primary nephrotic syndrome (PNS) is a disease characterized by the presence of nephrotic range proteinuria, clinical edema, hypoalbuminemia, and hyperlipidemia. It is one of the most common glomerular diseases in children, which accounts for 2 - 7 cases for every 100,000 children per year (1). Children with PNS have the secondary immunodeficiency showing the susceptibility to infection for their hypogammaglobulinemia and usage of glucocorticoid therapy. Respiratory tract infections (RTI) are common complications of PNS (2, 3). Infections not only reduce the efficacy of glucocorticoid treatment but also lead to prolonged duration and frequent recurrence in PNS (4, 5). The pathogenic spectrum and their susceptibility to antibiotics in RTI change over time, which increases the difficulty of anti-infection treatment. Therefore, prediction of the causative pathogens and their drug susceptibility before detecting results of respiratory pathogens in cases of PNS with RTI is needed to improve disease outcomes. There have been some researches on the etiology of RTI in China and abroad (6-8), but comprehensive studies on the etiology of RTI in children with PNS are scarce in the recent past.
2. Objectives
To improve the curative effect of PNS complicated with RTI, the clinical data of 2740 children with PNS who were inpatients in our hospital from 1.1.2010 - 12.13.2014 were analysed retrospectively in this study.
3. Methods
3.1. Clinical Data
2740 children diagnosed with PNS hospitalized in Children’s Hospital of Chongqing Medical University during 01.01.2010 - 12.31.2014 were enrolled in this study. Diagnosis of PNS referred to the 2012 KDIGO clinical practice guideline for glomerulonephritis (9).
RTI were divided into community-acquired respiratory tract infection (CARTI) and hospital-acquired respiratory tract infections (HARTI) according to the time of onset or divided into upper respiratory tract infection (URTI) and lower respiratory tract infection (LRTI) according to the site of infection. Definition and criteria refer to Zhu Futang textbook of pediatrics (10). CARTI, RTI occur before or within 48 hours of hospitalization; HARTI, RTI occur after 48 hours of inpatient; URTI, acute infections occur above the larynx, including the nose and pharynx of the upper respiratory tract; LRTI, acute infections occur below the larynx.
For recruited patients, nasopharyngeal aspirates (NPAs) and blood samples were collected on hospital admission after obtaining informed consent for the detection of common respiratory viruses, bacteria, and other atypical pathogens using current laboratory diagnostic tests.
NPAs were preserved in virus transport medium immediately after collection, and stored at -80°C prior to testing. Viral DNA and RNA were extracted from 200 mL of the NPA and eluted in 62 mL of AE Buffer by using QIAamp MinElute Virus Spin Kits (QIAGEN, Hilden, Germany). The complementary DNA sample was synthesized by using Super-Script First-Strand Synthesis system for reverse transcription polymerase chain reaction (RT-PCR) (Invitrogen, Camarillo, CA). All samples were screened by RT-PCR or PCR for common respiratory viruses using standard methods. Qualitative and semiquantitative cultures for bacteria were performed immediately using standard microbiological methods. Macroscopically distinct colonies of the samples were isolated in pure culture, and standard methods were used for identification, typing with sensitivity patterns.
Comparisons between different groups were performed using the chi-square test, and P ⟨ 0.05 was considered significant.
4. Results
4.1. General Information
Children with PNS complicated with RTI counted for 78.91% of our cases, totalling 2162 cases. The average age of the 2095 children with PNS and CARTI was 5.71 ± 3.69 years. The average age of the 67 children with PNS and HARTI was 5.8 ± 4.05 years. A total of 928 cases among the 2162 children with PNS and RTI underwent etiological examination; the examination rate was 42.92%, and the positive rate was 56.57% (525 cases).
4.2. The Pathogen Distribution of CARTI and HARTI in Cases with PNS
In cases of PNS with CARTI, 579 cases underwent sputum culture, and 208 cases (35.92%) were positive. A total of 270 strains of bacteria were isolated, among which there were 163 (60.37%) Gram-negative strains and 107 (39.63%) Gram-positive strains. Viruses, atypical pathogens and fungi were detected in 254, 117, and 12 of the specimens, respectively. The most common pathogen was Coxsackie virus, followed by Mycoplasma pneumoniae, Respiratory syncytial virus, Streptococcus pneumoniae, and Moraxella catarrhalis (Table 1).
| CARTI | HARTI | P Value | |||
|---|---|---|---|---|---|
| Strains (Strain) | Positive Rate, % | Strains (Strain) | Positive Rate, % | ||
| Bacteria | |||||
| Moraxella catarrhalis | 55 | 9.5 | 4 | 13.79 | 0.659 |
| Haemophilus parainfluenzae | 35 | 6.04 | 1 | 3.45 | 0.861 |
| Haemophilus influenzae | 24 | 4.15 | 1 | 3.45 | 1.000 |
| Unidentified Gram-negative bacteria | 21 | 3.63 | 2 | 6.9 | 0.688 |
| Klebsiella pneumoniae | 12 | 2.07 | 0 | 0 | 1.000 |
| Pseudomonas putida | 4 | 0.69 | 0 | 0 | 1.000 |
| Acinetobacter baumannii | 4 | 0.69 | 0 | 0 | 1.000 |
| Pseudomonas aeruginosa | 3 | 0.52 | 2 | 6.9 | 0.02 |
| Escherichia coli | 1 | 0.17 | 1 | 3.45 | 0.093 |
| Streptococcus pneumoniae | 65 | 11.23 | 5 | 17.24 | 0.489 |
| Unidentified Gram-positive bacteria | 26 | 4.49 | 4 | 13.79 | 0.069 |
| Staphylococcus aureus | 16 | 2.76 | 0 | 0 | 1.000 |
| other | 4 | 0.68 | 0 | 0 | 1.000 |
| Viruses | |||||
| Coxsackie virus | 121 | 37 | 5 | 71.43 | 0.143 |
| Respiratory syncytial virus | 79 | 17.25 | 6 | 30 | 0.246 |
| Adenovirus | 46 | 8.2 | 0 | 0 | 0.438 |
| Cytomegalovirus | 30 | 7.58 | 1 | 10 | 0.552 |
| Parainfluenza virus 3 | 19 | 5.12 | 0 | 0 | 1.000 |
| EB virus | 12 | 3.63 | 0 | 0 | 1.000 |
| Influenza virus A | 12 | 3.23 | 1 | 6.25 | 0.427 |
| Influenza virus B | 4 | 1.08 | 1 | 6.25 | 0.192 |
| Parainfluenza virus 1 | 3 | 0.81 | 1 | 6.25 | 0.156 |
| Herpes simplex virus | 1 | 0.86 | 0 | 0 | - |
| Atypical pathogens | |||||
| Mycoplasma pneumoniae | 112 | 23 | 4 | 28.57 | 0.868 |
| Chlamydia | 5 | 2.04 | 0 | 0 | 1.000 |
| Fungi | |||||
| Candida albicans | 8 | 1.38 | 2 | 6.9 | 0.078 |
| Unknown fungus | 3 | 0.52 | 0 | 0 | 1.000 |
| Candida tropicalis | 1 | 0.17 | 0 | 0 | 1.000 |
The Strains and Positive Rates of Respiratory Pathogens in Cases of PNS with CARTI and HARTI
In cases of PNS with HARTI, there were 15 positive specimens in 29 specimens for bacterial culture, with a positive rate of 51.72%. 11 strains of Gram-negative bacteria (55.00%) and 9 strains of Gram-positive bacteria (45.00%) were isolated. Viruses, atypical pathogens and fungi were detected in 10, 4, and 2 of the specimens, respectively. The most common pathogen was Coxsackie virus, followed by Respiratory syncytial virus, Mycoplasma pneumoniae, Streptococcus pneumoniae, and Moraxella catarrhalis (Table 1).
The overall positive rate of bacteria was lower in CARTI (35.92%) than HARTI (51.72%), but the difference was not statistically significant (P > 0.05). There was significant difference in the positive rates of Pseudomonas aeruginosa between 2 groups (P = 0.02), which indicated that opportunistic infections are more common in HARTI. Besides, the positive rates of other pathogens were not significantly different between the two groups (P > 0.05).
4.3. The Pathogen Distribution of URTI and LRTI in Cases with PNS
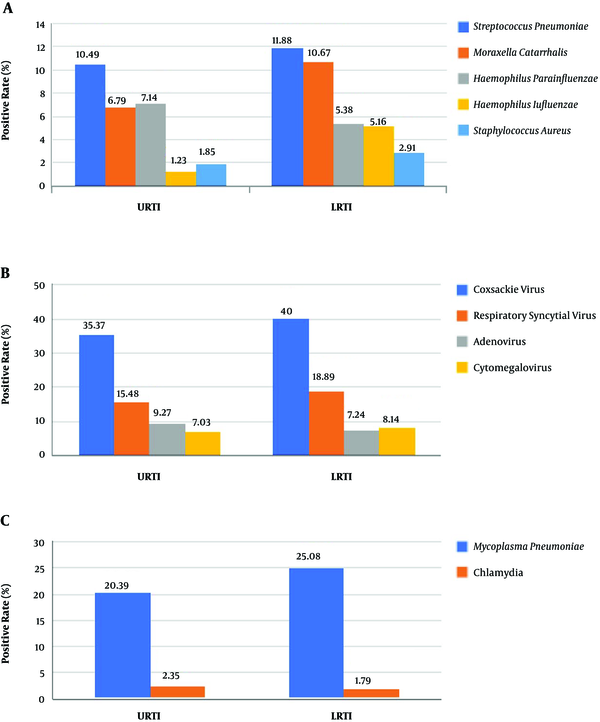
Among the cases of PNS with CARTI, there were 1317 cases of URTI (62.86%) and 778 cases of LRTI (37.14%). In URTI, the most common pathogen was Coxsackie virus, followed by Mycoplasma pneumoniae, Respiratory syncytial virus, Streptococcus pneumoniae, and Adenovirus (Figure 1). In LRTI, the most common pathogen was Coxsackie virus, followed by Mycoplasma pneumoniae, Respiratory syncytial virus, Streptococcus pneumoniae, and Moraxella catarrhalis (Figure 1).
The distribution of the respiratory pathogens varied batween URTI and LRTI (Figure 1). The positive rate of Haemophilus influenzae was considerably lower in URTI than LRTI, which was significantly different (P = 0.031). The positive rates of other pathogens shown in Figure 1 differed between 2 groups, but the results weren’t statistically significant (P > 0.05).
4.4. Analysis of Antibiotic Resistance of Bacteria
In cases of PNS with CARTI, 49 strains of extended-spectrum β-lactamase (ESBL) producing bacteria (18.15%) were isolated among 270 strains of bacteria, including 39 strains of Gram-negative bacteria and 10 strains of Gram-positive bacteria. In cases of PNS with HARTI, 3 strains of ESBL producing bacteria (15.00%) were isolated among 20 strains of bacteria and all were Gram-negative bacteria. The most common ESBL producing bacterium was Moraxella catarrhalis. Table 2 compares different ESBL producing strains and their positive rates between CARTI and HARTI.
| Bacteria | CARTI | HARTI | P Value | ||
|---|---|---|---|---|---|
| Strains (Strain) | Rate, % | Strains (Strain) | Rate, % | ||
| Gram-negative bacteria | 39 | 23.93 | 3 | 27.27 | 1.000 |
| Moraxella catarrhalis | 29 | 52.73 | 1 | 25 | 0.580 |
| Haemophilus influenzae | 7 | 29.17 | 1 | 100 | 0.320 |
| Haemophilus parainfluenzae | 1 | 2.86 | 0 | 0 | 1.000 |
| Enterobacter aerogenes | 1 | 100 | 0 | 0 | - |
| Escherichia coli | 1 | 100 | 1 | 100 | 1.000 |
| Gram-positive bacteria | 10 | 9.35 | 0 | 0 | 1.000 |
| Staphylococcus aureus | 10 | 62.5 | 0 | 0 | - |
ESBL Producing Strains and EBSL Positive Rates
In cases of PNS with CARTI, the sensitive rates of Gram-negative ESBL producing bacteria to carbapenems, gentamicin, levofloxacin, amoxicillin/clavulanate potassium, ampicillin/sulbactam, and ciprofloxacin were more than 90.00% (Table 3). Gram-positive ESBL producing bacteria were highly sensitive to glycopeptides, linezolid, quinolones, rifampicin, Macrodantin, mupirocin-HL, quinupristin/dalfopristin, and amoxicillin/clavulanate potassium with a sensitive rate more than 80.00% (Table 4). There was no statistical significance to analyse the antibiotic susceptibility and resistance of ESBL producing bacteria in HARTI because of the small sample size.
| Antibiotics | Susceptible (Ratio %) | Resistant (Ratio %) |
|---|---|---|
| Imipenem | 100 | 0 |
| Meropenem | 100 | 0 |
| Gentamicin | 100 | 0 |
| Levofloxacin | 100 | 0 |
| Ceftriaxone | 100 | 0 |
| Rifampicin | 97.3 | 0 |
| Ceftazidime | 94.74 | 5.26 |
| Amoxicillin/clavulanate | 92.31 | 7.69 |
| Ampicillin/sulbactam | 92.31 | 7.69 |
| Ciprofloxacin | 92.31 | 7.69 |
| Cefotaxime | 92.31 | 7.69 |
| Cefuroxime | 91.89 | 5.41 |
| Cefprozil | 82.35 | 17.65 |
| Cefaclor | 50 | 28.57 |
| Cefepime | 50 | 50 |
| Chloramphenicol | 82.05 | 15.38 |
| Azithromycin | 77.14 | 22.86 |
| Aztreonam | 69.23 | 30.77 |
| Tetracycline | 52.63 | 44.74 |
| Piperacillin/sulbactam | 50 | 0 |
| Piperacillin | 0 | 100 |
| Ampicillin | 0 | 97.44 |
| Trimethoprim/ Sulfamethoxazole | 33.33 | 66.67 |
| Cefazolin | 0 | 100 |
Susceptibility and Resistance of Gram-Negative ESBL-Carrying Bacteria in CARTI
| Antibiotics | Susceptible (Ratio %) | Resistant (Ratio %) |
|---|---|---|
| Vancomycin | 100 | 0 |
| Linezolid | 100 | 0 |
| Teicoplanin | 100 | 0 |
| Amikacin | 100 | 0 |
| Ciprofloxacin | 100 | 0 |
| Rifampicin | 100 | 0 |
| Macrodantin | 100 | 0 |
| Mupirocin-HL | 100 | 0 |
| Quinupristin/dalfopristin | 100 | 0 |
| Amoxicillin/clavulanate | 80 | 20 |
| Oxacillin | 77.78 | 22.22 |
| Penicillin | 0 | 100 |
| Ampicillin | 0 | 100 |
| Trimethoprim/sulfamethoxazole | 70 | 30 |
| Trimethoprim | 70 | 20 |
| Tetracycline | 62.5 | 25 |
| Gentamicin | 60 | 40 |
| Tobramycin | 60 | 40 |
| Clindamycin | 30 | 70 |
| Erythromycin | 30 | 70 |
| Cefoxitin | 20 | 20 |
| Fusidic acid | 20 | 0 |
| Mupirocin | 20 | 0 |
Susceptibility and Resistance of Gram-Positive ESBL-Carrying Bacteria in CARTI
5. Discussion
Owing to the substantial loss of protein in urine, the immunoglobulin of children with PNS stays at a very low level. Infection could occur in every system. RTI is one of the most common complications and has become a significant cause of relapse and death. In this study, the incidence rate of PNS complicated with RTI was 78.91%, which was higher than the incidence rate (72%) in Saudi Arabia reported by Alfakeekh et al. (1).
Our data showed that preschool and school-age children were likely to develop an RTI, while Wei et al. (2) reported that children under 10 were at a higher risk than children older than 10 in Taiwan. Different susceptible ages might vary in different times and regions. The main infection site of PNS complicated with RTI was the upper respiratory tract, which was similar to the findings reported in Japan (11). Therefore, we need to strengthen the management and screening of RTI in preschool and school-age children with PNS.
This study showed that the pathogen spectrum differed between cases of PNS with CARTI and with HARTI. The three most common bacteria in cases of PNS with CARTI were Streptococcus pneumoniae, Moraxella catarrhalis, and Haemophilus parainfluenzae. In the same period, the three most common bacteria in other cases of CARTI without PNS in local areas were Haemophilus parainfluenzae, Streptococcus pneumoniae and Moraxella catarrhalis (6), which was similar to our study. In addition, Streptococcus pneumoniae was the most common bacterium in cases of PNS with RTI in our centre, which was different from the main bacterium in hemodialysis patients reported by Gupta V (12), Staphylococcus aureus. This finding indicated that children with different kidney diseases developed RTI caused by different pathogens.
The most common virus in cases of PNS with CARTI was Coxsackie virus, followed by respiratory syncytial virus and adenovirus, while the most common virus in cases of PNS with HARTI was Coxsackie virus, followed by respiratory syncytial virus and cytomegalovirus. In the same period, the most frequently detected viruses in other cases of CARTI without PNS in local areas were respiratory syncytial virus, parainfluenza virus, and influenza virus (6), which was different to our study. The constitution of viruses in cases of PNS with RTI was different from the constitution of viruses in cases of RTI in children in eastern and southern China (13, 14). Our data showed that Coxsackie virus was the most common virus in cases of PNS with RTI, which was different from the respiratory syncytial virus suggested by foreign literature (15, 16). We considered that the main cause was age. Most of the children in our study were preschool- or school-age children. Coxsackie virus infection is likely to occur in children younger than 6, and respiratory syncytial virus infection is likely to occur in infants. Cytomegalovirus is one of the most common pathogens in infectious disease of infants and toddlers. The main target organ is the liver, but it can also lead to pulmonary infection. According to Doan et al. (17), cases of cytomegalovirus pneumonia often occur in infants under 3 months, indicating that cytomegalovirus is not the main pathogen in older children with RTI. Cytomegalovirus pneumonia should be diagnosed by corresponding signs in physical examination and CMV DNA quantitation in bronchoalveolar lavage fluid (18). However, cytomegalovirus in our patients was not detected in bronchoalveolar lavage fluid, it was detected by ELISA or PCR analysis of blood samples. We did not target the respiratory system to detect cytomegalovirus, so the results were not specific enough. Therefore, cytomegalovirus was not regarded as the main virus in cases of PNS with RTI in this study.
In this study, Candida albicans was the main fungus in cases of PNS with RTI, which was a similar result to that reported by Liu P (7).
Mycoplasma pneumoniae is a common pathogen in cases of CARTI in children. Previous studies show that the detected rate of Mycoplasma pneumoniae ranges from 8% - 35% in CARTI (7, 8, 19). In our data, the infection rate of Mycoplasma pneumoniae was 23% in cases of PNS with CARTI and 28.57% in cases of PNS with HARTI, and there were no significant differences between them (P > 0.05). Therefore, we suggest that the detection of Mycoplasma pneumoniae should be a routine test in cases of PNS with RTI.
According to this study, the distribution of bacteria differed between cases of URTI and LRTI in PNS with RTI. Therefore, we need to differentiate the location of RTI for empirical therapy in cases of PNS with RTI.
In this study, the ESBL positive rate of Gram-negative bacteria in cases of PNS with CARTI was 23.93%, which was obviously higher than that of Gram-negative bacteria (9.35%). Because the production of ESBL is mainly the resistance mechanism of Gram-negative bacteria.
In cases of PNS with HARTI, the ESBL positive rate of Gram-negative bacteria was 27.27%, which was not significantly different from that in cases of PNS with CARTI (P > 0.05). No ESBL producing bacteria were isolated among 9 strains of Gram-positive bacteria, which might be related to the small sample size of cases of HARTI.
According to the antibiotic susceptibility tests of drug-resistant strains, Gram-negative ESBL producing bacteria in cases of PNS with CARTI were highly sensitive to carbapenems, gentamicin, quinolones, rifampicin, ampicillin/sulbactam, and amoxicillin/clavulanate potassium. With a view to drug toxicity and price, amoxicillin/clavulanate potassium and ampicillin/sulbactam can be the first-line drugs for Gram-negative ESBL producing bacteria in cases of PNS with CARTI. Gram-positive ESBL producing bacteria were relatively sensitive to amoxicillin/clavulanate potassium. However, some β-lactamase inhibitors cannot completely inhibit ESBLs. If amoxicillin/clavulanate potassium and ampicillin/sulbactam are ineffective, we can choose carbapenems (20).
This study has some limitations. First, some common respiratory pathogens, such as human rhinovirus, human bocavirus, and human coronavirus, were not included in our study, which may lead to underestimation of the viral burden. Second, our findings can only be representative of children with PNS in local area, our findings may not be generalizable to other regions. Finally, we studied only hospitalized children, and a study of outpatients might have produced different results.
5.1. Conclusions
In conclusion, CARTI was more common than HARTI in cases of PNS with RTI, and URTI was more common than LRTI. Children of preschool and school age with PNS were more likely than younger or older children to develop an RTI. In children with PNS, the etiology differed between CARTI and HARTI as well as URTI and LRTI. Therefore, it is necessary to differentiate these features for empirical therapy. And we need to complete pathogen detection without delay. If the pathogen was bacterial, amoxicillin/clavulanate potassium could be the first choice before sputum culture results are available. We need to control infection as soon as possible to reduce the recurrence rate and improve the prognosis of children with PNS.