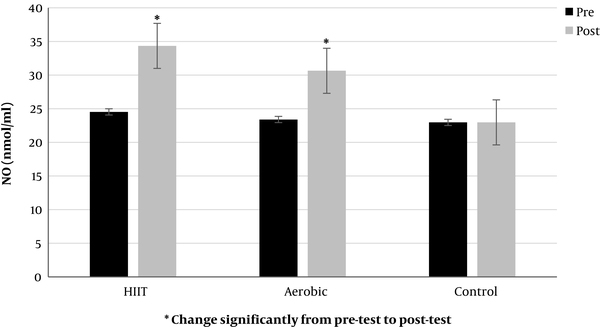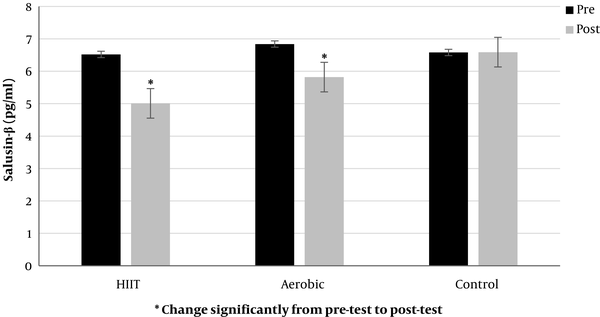1. Background
The prevalence and severity of childhood obesity has increased dramatically over past 50 years (1). Obese children are at a significantly increased risk of developing cardiometabolic diseases (2). Any degree of obesity can be considered as an independent risk factor for development atherosclerosis and an emphasis on presence of arterial malformations in children (3).
Salusins are a new class of bioactive peptides (4) that play an important role as endogenous modulators of atherogenesis (5). Kolakowska et al (6) in their study on 88 children and adolescents shown that serum salusin-β positively correlated with TG level and TG/HDL ratio. Grzegorzewska et al. (7) also showed a negative correlation between salusin-α and LDL.
Obese children show greater endothelial dysfunction and arterial stiffness than healthy peers (8). These findings are important because they indicate that endothelial dysfunction is one of early stages of cardiovascular disease and considered as one of primary symptoms atherosclerosis (9). Endothelial dysfunction is most likely due to reduced access to nitric oxide (NO) (10). Endothelial NO depletion is one of first primary and key injuries associated with onset of atherosclerosis (9). Animal and human studies have reported reduced bioavailability of NO in obesity (11).
While traditional interventions designed to increase physical activity and improve health have principally used moderate-intensity continuous exercise (12), the relevance of such programs to the patterns of high-intensity interval training for children has been questioned (13). Consequently, high-intensity interval trainings have recently been investigated as a potentially potent and time-efficient form of physical activity and health promotion (14).
2. Objectives
According to studies to date, efficacy of HIIT to improve pre-atherosclerotic and anti-atherosclerotic components has not been evaluated in obese and overweight children. Therefore, the present study did examine effect of two chronic exercise protocols on pre-atherosclerotic and anti-atherosclerotic biomarkers levels in overweight and obese children.
3. Methods
3.1. Contributors
This study was approved by the Research Ethics Committee of Kermanshah University of Medical Sciences (IR.KUMS.REC.1397.936). Before the involvement in the study, participants and their parents signed an informed consent in accordance with the international ethical standards, especially the Helsinki Statement (15) and a medical history review questionnaire and a referral form to the physician to check for puberty. Forty-five obese and overweight subjects (age: 11.06 ± 0.98 years, BMI: 25.12 ± 1.28 kg/m2), which were volunteered from 20 schools participated in this study conditionally. None of subjects participated in any regular and organized physical activity in 6 months prior to study, except for school exercise time.
Subjects did not use any medication or treatment for obesity, chronic illness, endocrine disorders or diabetes that may impair progress of study. Subjects could be voluntarily excluded from study for any possible reason, including personal, acute infectious disease, and acute training-related injuries. During the intervention period, all participants were asked to maintain their normal diet and not to take any medications during the week prior to blood sampling.
3.2. Anthropometric Measurements
Barefoot and least clothes their weights were measured using a digital scale (Tanita, Tokyo, Japan) with an accuracy of 0.1 kg. Subjects heights were measured in centimeters (Model 217 height rod; Seca, Hamburg, Germany). Waist and hip circumference were measured using a non-elastic tape meter without imposing any pressure on body with 0.1 cm accuracy. Body fat percentage (%BF) and lean body mass were measured using caliper (Harpenden model) and tweaking technique in three areas of back of arm, under shoulder blade and leg, on right side of subject’s bodies. To eliminate individual error, all measurements were by one person.
3.3. Testing Process
The maximum test to measure maximal aerobic speed (MAS) was performed in morning after a standard breakfast (containing approximately %55 carbohydrate, %33 fat, and %12 protein). Subjects performed test in an optimal space of 20-meter (suggested width 10-meter) marked by conical barriers. After necessary physical and mental fitness subjects were deployed at end of one of 20-meter marked lanes and moved to end of March 20-meter with first sound so that they could hear second sound at end of March. Subjects adjusted their speeds and increased their running speed until they were unable to continue test, as number of sweep cycles increased and time between two acoustic signals decreased. The baseline speed was assumed to be 8 km.h-1 and 0.5 km.h-1 for each passing minute. The speed maintained during last step corresponds to MAS.
3.4. Practice Protocol
Subjects were randomly assigned to a HIIT group (n = 15) that trained at 100% - 110% MAS (Table 1); an aerobic group (n = 15) who trained at 40% - 70% HRR (Table 1) and a control group without exercise. Exercise groups (aerobic and HIIT) trained 3 sessions per week on non-consecutive days according to their training protocols for 12 weeks. Training sessions included three parts: Standard warm-up section, main body of exercise and cooling part at end of training session. After consent, all participants completed baseline testing at week 0 and post-testing at week 12 of the study. Testing at two time points was similar. To ensure that subjects heart rate was within normal range and that no adverse effects of their heart rate occurs at training sessions, subjects were monitored with Germany Beurer PM 15 Cardio-tachometer.
| HIIT Group Exercise Protocol | ||||||
|---|---|---|---|---|---|---|
| Weeks | Warm-Up | Dynamic Stretching | Exercise | Recovery Between Training Periods | Cooling Down | Overall Time |
| 1st to 4th | 10 | 8 | 3 × (10 × 10 seconds); 100 maximum aerobic speed - active rest | 3 | 8 | 45 |
| 5th to 8th | 10 | 8 | 3 × (10 × 10 seconds); 105 maximum aerobic speed - active rest | 4 | 8 | 45 |
| 9th to 12th | 10 | 8 | 3 × (10 × 10 seconds); 110 maximum aerobic speed - active rest | 4 | 8 | 45 |
| Aerobic Group Exercise Protocol | ||||||
| Week | Warm-Up | Dynamic Stretching | Exercise | Cooling Down | Overall Time | |
| 1st to 4th | 5 | 5 | 30 minutes of aerobic exercise; 40 to 50 percent of saved heart rate | 5 | 45 | |
| 5th to 8th | 5 | 5 | 30 minutes of aerobic exercise; 50 to 60 percent of saved heart rate | 5 | 45 | |
| 9th to 12th | 5 | 5 | 30 minutes of aerobic exercise; 60 to 70 percent of saved heart rate | 5 | 45 | |
HIIT and Aerobic Groups Exercise Protocola
3.5. Biochemical Analysis
Blood samples were taken from subjects after approximately 12 hours of overnight fasting in 7:00 a.m. to 8:00 a.m. The collected blood samples were poured into heparinized lithium tubes and immediately centrifuged at 4°C. Serum and plasma were frozen in several micro-tubes after separation at -80°C until in vitro analysis.
Serum levels of salusin-α and salusin-β were measured by ELISA using Phoenix Pharmaceutical Kit, Burlingame, USA. Serum levels of NO were determined using a human NO assay kit, manufactured by Germany, by microplate Griss reaction. TG levels were measured enzymatically using a MAN kit, manufactured by Iran. HDL Levels was measured by Autoanalysis method and using Pars Test Kit, manufactured by Iran.
3.6. Statistical Analysis
Data were analyzed using SPSS software version 24 (P ≤ 0.05). Kolmogorov-Smirnov test was used to determine normal distribution of data. Since data had normal distribution, ANOVA with repeated measures was used to examine intergroup differences, and also to examine pre-test to post-test intragroup effects in each group was used paired t-test.
Since data were not a normal distribution, Wilcoxon test was used to examine intragroup differences and the nonparametric Cruskal-Wallis test for intergroup differences. The Mann-Whitney test was also used to examine delta of experimental groups.
4. Results
Physical, physiological and blood variables of subjects in all three groups at beginning and end of 12-week study are summarized in Table 2. Table 2 shows that in both training groups (HIIT and aerobic, respectively) from pre-test to post-test body weight (P = 0.000, P = 0.025), BMI (P = 0.000, P = 0.029), %BF (P = 0.000, P = 0.009) and WHR (P = 0.000, P = 0.041) were significantly reduced, but Intergroup reviews showed that body weight loss (-0.76 kg in HIIT and -0.17 kg in aerobic group), BMI (-0.33 kg in HIIT and -0.07 kg in aerobic group), %BF (-1.25 kg in HIIT and -0.02 kg in aerobic group) and WHR (-0.015 kg in HIIT and -0.002 kg in aerobic group) were significantly higher in HIIT group than in aerobic group. However, body weight (P = 0.038) and BMI (P = 0.037) were significantly increased in control group, but increased %BF (P = 0.415) and WHR (P = 0.056) was not significant.
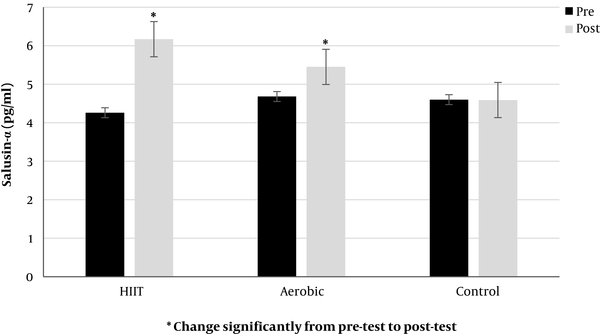
On the other hand, Table 2 shows that in both training groups (HIIT and aerobic, respectively) from pre-test to post-test serum salusin-α levels (P = 0.000, P = 0.000, Figure 1) and NO (P = 0.000, P = 0.000, Figure 2) were significantly increased, but intergroup reviews showed that increase in Salusin-α (1.90 mL/min in HIIT and 0.77 mL/min in aerobic group) and NO (9.81 mL/min in HIIT and 7.24 mL/min in aerobic group) were significantly higher in HIIT group. However, levels of Salusin-α (P = 0.116) and NO (P = 0.811) in control group decreased but were not significant.
| HIIT (N = 15) | Aerobic (N = 15) | Control (N = 15) | ||||
|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | |
| Age, y | 11.13 ± 0.99 | 10.86 ± 1.06 | 11.20 ± 0.94 | |||
| Height, cm | 148.53 ± 4.40 | 148.26 ± 4.49 | 145.26 ± 4.71 | |||
| Weight, kg | 56.00 ± 4.51 | 55.24 ± 4.55b, c | 55.06 ± 3.75 | 54.90 ± 3.84c | 54.20 ± 4.45 | 54.38 ± 4.44d |
| BMI, kg/m2 | 25.33 ± 1.01 | 25.00 ± 1.06b, c | 25.01 ± 0.69 | 24.94 ± 0.73d | 25.02 ± 1.89 | 25.10 ± 1.85d |
| WHR, cm | 0.95 ± 0.02 | 0.93 ± 0.02b, c | 0.946 ± 0.03 | 0.944 ± 0.03d | 0.950 ± 0.02 | 0.956 ± 0.01d |
| %BF | 28.04 ± 1.46 | 26.78 ± 1.59b, c | 27.87 ± 1.06 | 27.85 ± 1.34c | 27.88 ± 1.06 | 27.93 ± 1.11 |
| Salusin-α, pg/mL | 4.26 ± 0.74 | 6.17 ± 0.70b, c | 4.68 ± 0.57 | 5.45 ± 0.62c | 4.60 ± 0.98 | 4.59 ± 0.99 |
| Salusin-β, pg/mL | 6.52 ± 0.87 | 5.01 ± 0.78b, c | 6.84 ± 0.85 | 5.82 ± 0.61c | 6.58 ± 0.75 | 6.59 ± 0.74 |
| Nitric oxide, nomL/mL | 24.53 ± 2.09 | 34.34 ± 2.69b, c | 23.40 ± 2.56 | 30.64 ± 2.56c | 22.97 ± 2.11 | 22.97 ± 2.11 |
| TG, mg/dL | 150.53 ± 31.38 | 117.00 ± 23.98b, c | 163.53 ± 18.48 | 143.00 ± 15.03c | 147.93 ± 28.68 | 149.13 ± 29.02 |
| HDL-C, mg/dL | 40.58 ± 1.53 | 43.33 ± 1.91b, c | 40.80 ± 1.88 | 43.07 ± 1.64c | 41.40 ± 1.88 | 41.00 ± 1.36 |
| TG/HDL, mg/dL | 3.68 ± 0.47 | 2.69 ± 0.57b, c | 3.99 ± 0.47 | 3.44 ± 0.40c | 3.58 ± 0.76 | 3.61 ± 0.71 |
Physical, Physiological and Blood Variables Before and After the Interventiona
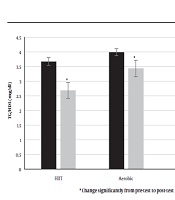
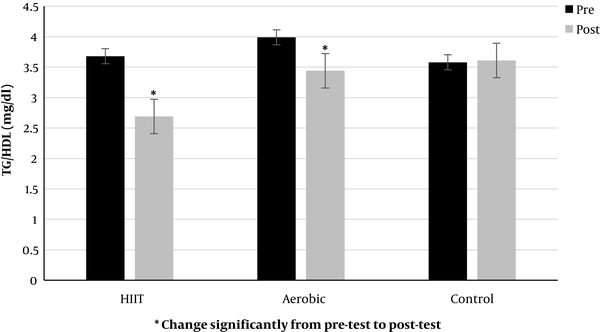
Also, in both training groups (HIIT and aerobic, respectively) from pre-test to post-test significantly decreased serum levels of salusin-β (P = 0.000, P = 0.000, Figure 3) and TG/HDL ratio (P = 0.003, P = 0.001, Figure 4), but intergroup reviews showed a decrease in salusin-β (-1.51 mL/min in HIIT and -1.02 mL/min in aerobic group) and TG/HDL ratio (-0.98 mL/min in HIIT and -0.49 mL/min in aerobic group) were significant in HIIT group. However, levels of salusin-β and TG/HDL ratio (P = 0.116) increased in control group, but it was not significant (P = 0.054).
5. Discussion
Salusins originally identified using bioinformatics analyses have been shown to act on the cardiovascular and endocrine systems. The salusin-α suppresses human foam cell formation and thus have anti-atherosclerotic effects. However, salusin-β stimulates the formation of human macrophage foam cell and accelerates the development of atherosclerosis (16). Salusin-α could suppress the expression of acyl-CoA: cholesterol acyltransferase-1 (ACAT-1), which stores cholesterol ester converted from free cholesterol and spontaneously increases during monocytic differentiation into macrophages (16). Therefore, salusin-α could suppresses foam cell formation by down-regulation of ACTA-1.
The anti-atherosclerotic effect of salusin-α has been supported by research on people with CVD, such that serum levels of salusin-α in subjects with coronary artery disease was significantly lower in compared to control subjects (16). After 4 - 8 weeks of salusin-α injection, process of atherosclerotic aortic injury was suppressed (17). Furthermore, serum salusin-α levels show a close negative correlation with the severity of atherosclerotic diseases. Salusin-α not only inhibits ACAT-1 expression and macrophage foam formation, but it also reduces serum total cholesterol levels, thereby exerting anti-atherosclerotic activities (17). These results, together with the clinical observation that decreased serum Salusin-α levels are associated with atherosclerosis in humans, leads to the hypothesis that Salusin-α has a protective effect against development of atherosclerosis (18).
Modulatory effects of salusin-β on atherosclerotic lesion formation have also been demonstrated in in vivo atherosclerosis models: apoE-deficientmice and LDL receptor deficient mice. Expression of salusin-β is increased in atherosclerotic lesions in LDL receptor deficient mice (19). Subcutaneous injection of salusin-β into LDL receptor-deficient mice aggravated atherosclerotic lesions, and this effect was associated with significantly increased serum LDL cholesterol levels (19). These data support the notion that salusin-βexerts systemic pro-atherogenic activity. Salusin-β will increase foam cell formation by increasing regulation of ACAT-1 (16). Overexpression of Salusin-β in atherosclerotic lesions in vascular tissue and improvement of vascular changes by salusin-β anti-body indicate a regulatory role of salusin-β in development or maintenance of atherosclerosis (16).
Decreasing salusin-β levels after a chronic period of training, especially HIIT, is a very important finding, as it indicates that present study has been successful in reducing a key pre-atherosclerotic factor with greater emphasis on HIIT. Therefore, HIIT can be a more prominent anti-atherosclerotic agent. Although reasons for HIIT being more successful than aerobic exercise is beyond scope of present study, it may be related to higher intensity of this exercise model. In this context, it has to be mentioned that during the intervention, subjects enjoyed the training sessions on the track (20) and were motivated to the challenge of reaching the cones at the requested time independently of the exercise intensity. This, has to be considered in designing training protocols for obese and overweight children, probably contributing to lower drop-out rates for larger samples of subjects compared to classical “boring” continuous running.
Increased resting NO levels may be due to shear stress applied to vascular network in long term. Shear stress is known as force exerted by bloodstream on walls of blood vessels. This shear stress elicits a response in vessel wall, characterized by release of endothelial mediators and in turn stimulates structural remodeling by activating gene expression and protein synthesis (21). Other important factors regulating vascular response to shear stress are blood flow characteristics (size and shape) and vascular tree anatomy (22).
Also, one of enzymes whose expression increases in response to shear stress is eNOS (23). Activation of endothelium integrin due to shear stress can phosphorylate more eNOS in serine 1179 which directly enhances eNOS activation and NO production. This has been shown to occur with a transient increase in transcription and a prolonged increase eNOS in mRNA (24). Increased eNOS transcription, although transient, is important, as it may underlie increase in eNOS induced by exercise training that by increase cardiac output enhancing eNOS expression (25). Previous studies have shown that exercise training increases nitric oxide synthase mRNA expression (26) and eNOS protein levels (27) in coronary arteries.
The precise mechanisms by which salusins affect fat metabolism and atherosclerosis are unclear, and further research is needed to address this issue (4). However, a significant decrease in TG/HDL ratio after 12 weeks of HIIT and aerobic exercise training in both intervention groups compared to pre-test in present study indicates usefulness of exercise activity, especially HIIT in reducing this proportion. Also, nonsignificant decrease in levels of salusin-α, nitric oxide and HDL plus nonsignificant increase in levels of salusin-β, TG and TG/HDL ratios in control subjects after 12 weeks of study indicate that living a sedentary lifestyle, physical inactivity and a change in lifestyle over time can make things worse for these children.
Reduction in Weight body and %BF were also observed in both training groups. This is consistent with findings that exercise training significantly reduces fat mass compared with dietary changes in obese and overweight subjects, even when exercise intensities were low (28). In this context, HIIT has been more effective despite same volume (45 minutes) and duration of training (12 weeks), as it has emerged as most effective exercise to reduce body mass in adolescents (29). HIIT had more favorable effects on improving anthropometric parameters. This finding supports idea that HIIT is more efficient in obtaining an optimal body composition. However, results reported in this study are consistent with results from previous studies that reported a significant improvement in body fat and body mass (30). On the other hand, it seems that duration of intervention (12 weeks) was sufficient to stimulate central physiological adaptations and that increased MAS chronically stimulated numerous cardiovascular and metabolic adaptations.
5.1. Conclusions
According to our findings, a 12-week training period (Aerobic and HIIT), especially HIIT, significantly increase salusin-α levels, as an anti-atherosclerotic agent and nitric oxide, as a vasodilator in obese and overweight children. Also, a 12-week training period (Aerobic and HIIT), especially HIIT, significantly decrease salusin-β levels, as a key pre-atherosclerotic factor and and TG/HDL ratio in obese and overweight children. Therefore, the use of HIIT seems to be considered as an important factor in preventing chronic diseases of passive lifestyle for obese and overweight children, so that the research findings the present confirm the anti-atherosclerotic effect of exercise training, especially HIIT. Then, to improve cardiometabolic health in obese and overweight children, we recommend regular physical activity with greater emphasis on HIIT.
5.2. Research Limitations
This study was cross-sectional with a relatively small sample size, which may cause errors in the correlations due to other factors not taken into account in the statistical analysis. In the present study, we were unable to accurately control nutrition because of inadequate access to subjects. The use of boy subjects and the impossibility of investigating gender-related changes in the research variables have led to the possibility of the findings of the research not being valid in the girl subjects. Also, due to some problems, it was not possible to monitor the psychological and stress conditions of the subjects during the study, especially during blood sampling. On the other hand, the unavailability of laboratories and precise physiological devices made us to use field tests to determine some parts of the research.