1. Background
Mycoplasma pneumoniae (M. pneumoniae) infection presents various clinical signs and symptoms, such as fever, sore throat, cough etc. (1). It is considered as an important factor for respiratory tract infection in children with clinical manifestations of upper respiratory infections, pneumonia and extrapulmonary manifestations (such as non-specific exanthem, encephalitis, etc.) (2). M. pneumoniae pneumonia (MPP) is one of the main causes leading to children's hospitalization. MPP accounts for approximately 10% to 40% of community-acquired pneumonia (CAP) cases in children (3-5). The epidemic cycle of MPP occurs every 3 - 5 years (6, 7). M. pneumoniae pneumonia occurs most frequently among school age children (older than 5 years) (3, 8). However, recent reports revealed that pre-school children even infants would be susceptible to M. pneumoniae infection and show clinical symptoms (9-11). MPP is traditionally described as mild and self-limited entity which is characterized by a persistent dry cough. However, recently many severe, fulminant or even fatal cases of MPP have been reported including asthma-like exacerbations in young children and infants (11, 12). So, it is very important to monitor the epidemiological characteristics of MPP among children in the treatment of M. pneumoniae.
2. Objectives
Our study, based on a large sample size, was to characterize the M. pneumoniae epidemic and evaluate epidemiological features in hospitalized children with M. pneumoniae pneumonia in Hangzhou, China.
3. Methods
3.1. Study Population
From July 2015 to June 2019, 71965 children were enrolled in this study. The samples should conform to all the following criterions: (1) children who were < 14 years old; (2) patients who were hospitalized in Children’s Hospital of Zhejiang University School of Medicine (the largest comprehensive center for pediatric health care in Zhejiang province, China, based in Hangzhou city); (3) patients who had been primarily diagnosed with pneumonia (13). The children with noninfective pneumonia (such as aspiration pneumonia, hypersensitivity pneumonia, etc.) were excluded from the study. In this study, we collected the respiratory tract samples (throat swab or sputum) from every object on the first day of hospitalization and performed SAT-MP (simultaneous amplification and testing of M. pneumoniae) assay. This study was approved by the Medical Ethics Committee of the Children’s Hospital of Zhejiang University School of Medicine.
3.2. Detection of M. pneumoniae
SAT-MP assay (Shanghai Rendu Biotechnology Co, Ltd) was a useful method to detect M. pneumoniae 16s rRNA, which was reported in our previous studies (14). The amplification process was performed using ABI 7500 detection system under conditions of 40 cycles of 42°C for 60 seconds.
3.3. Statistical Analysis
The results were analyzed using SPSS software (version 20.0). χ2 test was performed to investigate the statistical differences. Two-tailed P value of less than 0.05 (P < 0.05) considered statistically significant.
4. Results
During July 2015 to June 2019, 71,965 samples (throat swab or sputum) were collected and tested by SAT-MP assay. 42350 were boys and 29615 were girls, yielding a male-to-female ratio of 1.43:1. 10206 (5598 from boys and 4608 from girls) samples were tested positive for M. pneumoniae, with an average positive rate of 14.2%. The positive rate in girls (15.6%) was higher than that in boys (13.2%, P < 0.05).
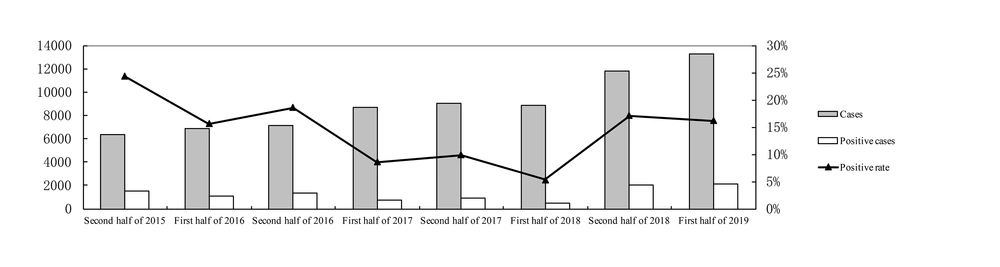
As shown in Figure 1, the highest positive rate of M. pneumoniae infection in the first half year rose up to 16.2% (2019) and the lowest dropped to 5.3% (2018) (P < 0.05). The highest positive rate of M. pneumoniae infection in the second half year rose up to 24.3% (2015) (P < 0.05), compared with the lowest rate of 9.8% (2017) (P < 0.05). The annual positivity rates of M. pneumoniae were 17.1%, 9.2%, 12.1% in 2016, 2017, and 2018 respectively.
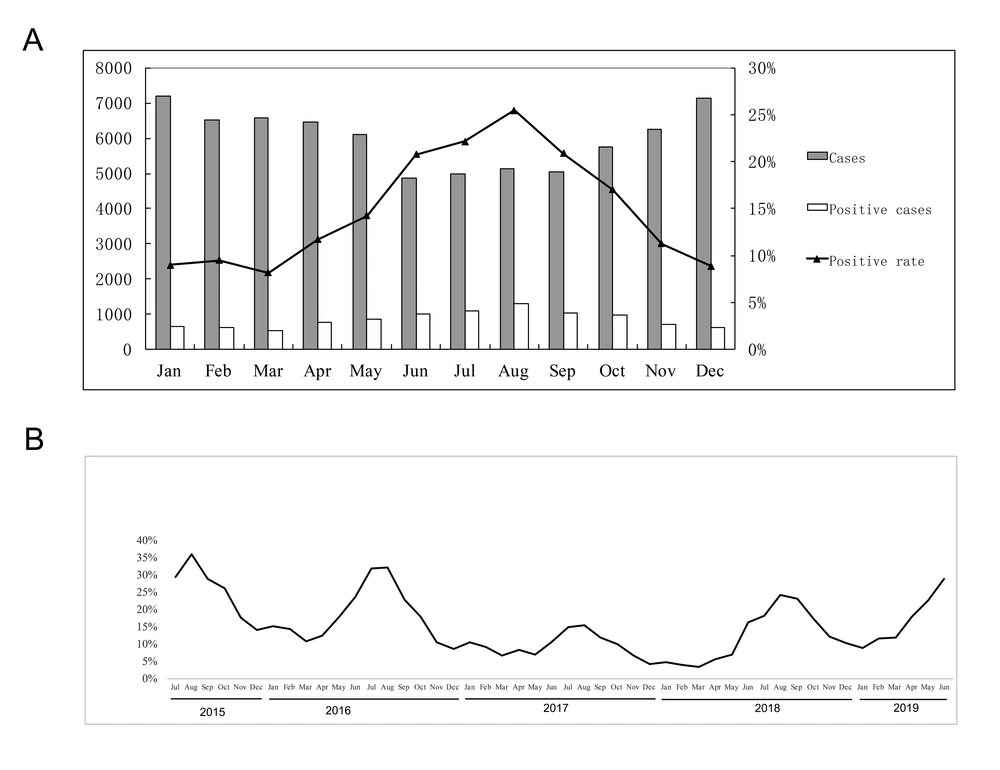
As shown in Figure 2A, monthly results during the whole study period showed the highest positive detection in August (25.5%), while the lowest in March (8.2%) (P < 0.05). The monthly positive rates of 2015 - 2019 are shown in Figure 2B. During the epidemic period, there was a peak of the M. pneumoniae positive rate in every summer.
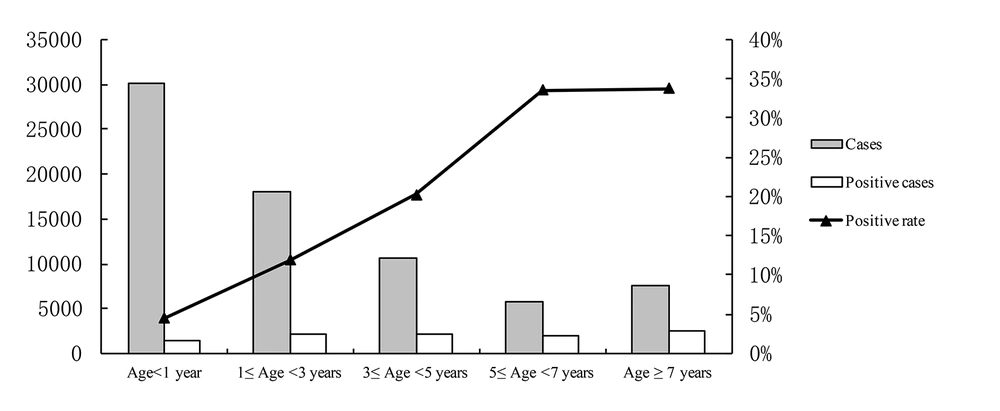
As shown in Figure 3, infants less than 1 year old showed the lowest infection rate (P < 0.05). The positive rate of MPP increased with age and reached the peak among children older than 5 years old (above 33%, P < 0.05). The infected rates in children of different age were 4.5% (0 - 1 year), 11.9% (1 - 3 years), 20.2% (3 - 5 years), 33.6% (5 - 7 years), and 33.8% (> 7 years), respectively.
5. Discussion
This is a study with large sample size monitoring M. pneumoniae in hospitalized children with pneumonia. In our study, 71,965 respiratory tract samples were surveyed. In this study, we observed that 14.2% (10206/71965) of hospitalized children with pneumonia were positive for M. pneumoniae, which was similar to the study in North China (15) and also comparable with the global incidence of 12% (range 11% - 15%) published by the Atypical Pathogens Reference Laboratory Database (16). Gao et al. reported the rate of MPP reaching 37.5% in pediatric patients with pneumonia in North China (17) which is higher than our study. In addition, we also observed that the rate of MPP in peak years increased, reaching to 38.6% in September 2019 (which is not shown in the article).
We used SAT-MP targeting M. pneumoniae 16s rRNA for detection of M. pneumoniae. There are two advantages in this method: (1) It has higher sensitivity, because 16s rRNA template is much more than the genome of M. pneumoniae in one copy of M. pneumonia (14, 18); (2) it suggests live M. pneumoniae infection, SAT-MP targets to 16s rRNA and its half life is much shorter than DNA (19). Compared with the results of DNA test, SAT-MP can give the true positive result of M. pneumoniae in early infection stage.
Several studies showed gender was not a risk factor for M. pneumoniae infection (20, 21). However, in our surveillance period, the positive rate of M. pneumoniae pneumonia was higher in girls compared with boys, which was in accordance with our previous study (22) and the study in North China (17). It indicated that girls are more susceptible to M. pneumoniae infection. Previous studies reported that M. pneumoniae pneumonia varied in occurrence rate over the years, showing an epidemic cycle of 3 - 5 years worldwide and persisted for 1 - 2 years (23, 24). In our study, we found that in the second half of 2015, the positive rate of M. pneumoniae reached 24.5%, indicating that 2015 was the year of M. pneumoniae epidemic. During the first few months of the second half of 2019, the positive rate of M. pneumoniae showed an upper trend (36.9% in July, 38.5% in August, 38.6% in September and 29.6% in October, not shown in this article), which suggested 2019 may be another year of M. pneumoniae epidemic. These results were similar to the epidemic patterns of MPP in Korea, with a cycle of 3 - 4 years (25).
In year-round or apart, summer (June to September) was the peak season for M. pneumoniae pneumonia in Hangzhou, with the highest occurrence in August. Previous studies also revealed that infections tend to be more common in summer and early autumn. A study in northern China revealed that M. pneumoniae infection was more frequent between July and November, with the same manifestation among children and adults (15). Another study in North China showed that autumn (September to November) was the peak season (17). But Kutty et al., following a two-and-half-year study, found that in the USA, M. pneumoniae infection was endemic without significant seasonal fluctuations (26). This may be due to the influence of different climates (22).
Our study indicated that children older than 5 years, with the incidence over 30%, were the most susceptible population. The findings are similar to many previous studies (3, 9, 10, 22, 27). However, in our study young children also have a high rate of M. pneumoniae infection in Hangzhou, with 20.2% among the 3 - 5 year-olds and 11.9% among the 1 - 3 year-olds. The positive rate of M. pneumoniae was 4.5% in infants. Thus, M. pneumoniae was considered as a cause for asthma-like exacerbations in young children and infants (11, 12). The clinical characteristics of positive M. pneumonia tests in population of infants and young children deserve constant attention in our future research.
5.1. Conclusions
The M. pneumoniae positive rate was higher in older hospitalized children and it had the highest prevalence in summer in Hangzhou. Since this article was defective with the comparatively short study period, in the future studies we will continue to monitor the epidemiological and clinical characteristics of M. pneumoniae and the prognosis of infants infected with M. pneumoniae.