1. Background
Alone in the United States annually six million children undergo surgeries under GA while 1.5 million of them are infants. Anesthesia benefits in pediatric surgeries including maintaining stable hemodynamic state, reduction of pain and anxiety, providing proper conditions for surgeon are not deniable. However recently the safety of GA in young children has been questioned (1, 2). Experimental studies have shown that early exposure of developing brain to general anesthesia results in neurodegenerative changes (3). The current available anesthetic drugs which act as N-methyl-D-aspartic acid receptor antagonists (NMDA) and γ-amino butyric acid modulator interfere with CNS development. Since all anesthetic drugs except of opioids and agonists act as above, GA and deep sedation can result in apoptotic neuro-degeneration. These agents affect primarily cortical regions through apoptotic phenomena (4). These changes cause deficits in different aspects of behavior. Indeed the findings of animal researches induce the concern that the mentioned risk might be also in human brain. Up to now human studies have discussed the risk of neurotoxicity related GA with controversial results (5). In spite of a number of researches with different outcome measures, (e.g. intelligence, academic achievements neuro- psychological statue biomarkers and neuro-imaging) that have assessed the GA harm in developing brain, there are a lot of gaps in our knowledge (6). Due to the lack of an agreement on the topic, whether GA causes neurodevelopment impairment, investigations continue to reach a definite answer (7). The concern regarding the risks of anesthesia agents is not restricted just to anesthesiologists’ society, it has involved other fields, United States Food and Drug Administration (FDA), the European Medicines Agency and even partly public society (8). A wide range of time from pregnancy up to 4 years have been considered as unsafe age for GA (9). In an experimental research, Fredriksson and Archer (10) examined the effects of induction of general anesthesia with ketamine on rodents and found that they developed hyperactivity which responded well to dextroamphetamine.
Although translation of the clinical significance of these data to human brain is difficult, it was supposed that this pattern may mimic human attention-deficit/hyper activity disorder (ADHD) in children (11). ADHD as the most common neuropsychological disease of children may continue into adulthood. The child suffers from uncontrolled impulses, motor restlessness and impaired ability to pay attention and concentrate (12).
The predisposing factors for this disease are not well known yet. However, available information strongly points to the important role of both gene and environment for clinical manifestation of ADHD (13). Due to the scarcely limited researches in our country and the importance of the issue, this study was planned.
2. Objectives
In the present study we investigated whether GA exposure before four years of age was associated with behavioral problems or not.
3. Methods
This case control study was conducted at pediatric psychology clinic of our institution and a pediatric neurology private clinic, from February to July 2019. Firstly the responsible resident of anesthesiology screened all the files of children who were referred to the mentioned centers and ADHD cases that were recently diagnosed were identified and sorted out.
Inclusion criteria consisted of newly diagnosed ADHD cases (within the last year) having a healthy brother or sister, whose parents were contactable and also accepted to enroll in the survey. An experienced pediatric psychiatrist and a pediatric neurologist identified our cases, according to the DSM-5 diagnostic criteria for ADHD.
3.1. Exclusion Criteria (Families with No Cooperation)
After sorting out the files, the resident of anesthesiology telephoned the families of these children and explained the aim of the survey. When they agreed to participate, a questionnaire was filled out through an almost 10 minute’s telephone interview for both ADHD cases and the healthy sister or brother. The parents were asked to answer the questions including the children’s history of exposure to GA in the first four years of age, the age of exposure, birth status; term or preterm and single or multi-exposure to GA. The main outcome of the study is development of ADHD when exposed to general anesthesia in childhood.
Finally the frequency distribution of GA exposure was compared between ADHD cases and controls and the data were compared between healthy and ADHD groups to find any association between receiving GA in the first four years of age and ADHD.
3.2. Sample Size
Based on a pilot study we found that 104 cases could be a proper sample size for this survey. From 30 ADHD cases, six (20%) had a history of general anesthesia exposure in the first four years of age. Study power was considered as 90%, Z = 1.96 and β-1 = 1.28. The used formula is presented below.
3.3. Ethics
After approval of the study protocol by the Ethics in Research Committee of the University (no.: IR.GUMS.REC.1397.524), informed consent was obtained from parents.
4. Results
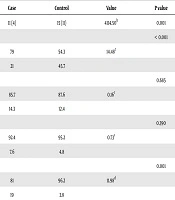
The data from 210 children were analyzed. Among 105 ADHD cases, 19% had a history of procedures requiring general anesthesia while it was 3.8% in control group. The characteristics of cases and controls are presented in Table 1. Comparing Comparing the two groups a significant difference was observed regarding the age of receiving GA (P = 0.004), gender (P < 0.001), the history of receiving GA (P = 0.001) and the number of anesthesia exposures (P = 0.001). Hence in order to adjust the variables with significant difference in univariate analysis, (sex, history of anesthesia exposure, number of anesthesia exposures and the age when received anesthesia), backward likelihood ratio method was applied and after three steps, age, sex remained statistically significant. According to logistic regression analysis, male gender (P = 0.003) OR 0.92 (95CI = 0.87 - 0.97) and age (P = 0.001) OR 3.11(95CI = 1.63 - 5.93) were significant predictors of early exposure to GA and ADHD development (Table 2).
| Case | Control | Value | P value | |
|---|---|---|---|---|
| Age, y | 11 (4) | 15 (11) | 4114.50b | 0.001 |
| Sex, % | < 0.001 | |||
| Male | 79 | 54.3 | 14.48c | |
| Female | 21 | 45.7 | ||
| Birth weight, % | 0.685 | |||
| Normal | 85.7 | 87.6 | 0.16c | |
| Low birth weight | 14.3 | 12.4 | ||
| Situation at birth, % | 0.390 | |||
| Term | 92.4 | 95.2 | 0.73c | |
| Preterm | 7.6 | 4.8 | ||
| History of anesthesia exposure, % | 0.001 | |||
| No | 81 | 96.2 | 11.98d | |
| Yes | 19 | 3.8 | ||
| Number of anesthesia exposures, % | 0.001 | |||
| Zero | 81 | 96.2 | 12.38d | |
| Once | 15.2 | 3.8 | ||
| > Once | 3.8 | 0 | ||
| Age when receiving anesthesia, % | P = 0.004 | |||
| One ye | 5.7 | 1.9 | 13.16d | |
| Two years | 5.7 | 0 | ||
| Three years | 4.8 | 1.9 | ||
| > Three years | 2.9 | 0 |
aValues are expressed as No. (%) or median (interquartile range).
bMann-Whitney U.
cPearson chi-square.
dFisher’s exact.
aR2 = 0.17 (Cox & Snell), 0.22 (Nagelkerke). Model χ2 (5) = 39.11, P < 0.001.
bConfidence interval.
cP = 0.003.
dP = 0.001.
Statistics analysis: Statistical package for social science (SPSS) version 16 software was used to analyze the data. According to Kolmogorov-Smirnov test the age distribution was not normal among cases and controls. Mann-Whitney U and chi-square tests were used. In multivariate analysis, we used logistic regression analysis for predicting our dichotomous outcome (ADHD). Statistical significance was considered as P < 0.05.
5. Discussion
Neurodevelopment abnormalities in ADHD cases have been described well. Studies have found that in these cases, hypofunction of N-methyl d-aspartate receptors induce inattention. Prefrontal cortex (PFC) is responsible for thoughts, analysis and regulating behavioral, emotion focus and attention. PFC helps to predict the outcomes of a behavior and determining right from wrong. This vital part of the brain is unregulated in ADHD cases and dendritic spine density in PFC significantly change (14). According to our search, it was the first study in Iran evaluating the association between early GA exposure and later behavioral disorders. Indeed, increasing interest in this topic as a big concern judged by numerous published articles, has not been observed in our country and the limited available studies indicate the lack of enough attention to the issue (15, 16). The main finding of this work was that children exposed to anesthetic agents in the first four years of age, had a higher incidence of ADHD than those without this history. In other words we supported previous studies which indicated the neurotoxicity of anesthetic drugs in developing brain. We found that the age of receiving GA, male gender, the history of receiving GA and the number of exposures were significantly associated with ADHD. However they could not be a strong predictor for this behavioral disorder. After our data was stratified by sex, we found a strong association between ADHD and male gender. Searching the current literature, some of them supported our findings and some other were in contrast. In line with our paper, Tsai et al. (17) in a birth cohort study reported that exposure to GA before the age of three years had an increased risk for later ADHD. DiMaggio et al. (18) in two retrospective studies found that exposure to GA in the first 3 years of age increased the risk of developmental or behavioral abnormalities. Ing et al. (19) in 2012 indicated that children who were exposed to GA in the first three years of age showed more language deficits than unexposed ones. Furthermore studies reported that neonates delivered by cesarean section under GA were more likely to develop behavioral deficits compared with those delivered vaginally without anesthesia (20). Flick et al. (21) 2011 demonstrated that early exposure to GA could be an independent risk for neurological disorders affecting both learning and behavior abilities. In contrast to our findings, in a pilot study Kalkman et al. (22) reported that there was a non-significant association between exposed children and non-exposed to GA before 24 months regarding behavioral disorders. Bartels et al. (23) did not report the mentioned association either. O’leary J et al. (24), reported that children who received GA before age 5 to 6 were at a higher risk of early neurodevelopment vulnerability and long term adverse outcomes. However they found that multiple exposure or age under 2 were not recognized as additional risks. Sprung et al. (11) studied the association between GA before 2 years of age and the development of ADHD. They found that children, who underwent repeated surgeries under GA, had a higher risk of development of ADHD. Opposite to this work, Ko et al. (25) in a retrospective matched-cohort study in Taiwan reported that there was no association between early life anesthesia exposure before three years of age and ADHD. Creagh et al. (26) also did not observe any positive correlation. Bong et al. (27) in a retrospective study found that the incidence of learning disability among children with a history of GA exposure before one year was 4.5 times greater than that of not exposed peers. As discussed above, a discrepancy among the findings of human studies is observed which could be justified by the differences regarding socioeconomic status, genetics, familial conditions, parenteral characteristics such as age, comorbidities, dosage and timing of GA, all of which might affect the results. Indeed the reason of this disagreement could be differences in study design such as choice of study population, sample size, the length of follow-ups, different assessment tools (e.g. intelligence, academic success, behavioral disorders) (28). The definition of ADHD and case selection strategies might be different among studies. In Sprung et al.’s study (11) the majority of cases came from schools, that had referred the children for behavioral problems and a questionnaire was filled out by teacher or parent. Tasi et al. (17) used a nationwide population-based sample and in our research we had a regional participation. Furthermore studies which select cases based on ICD-9-CM code 314.01 that presents a combined type of disease, may miss ADHD cases who clinically express hyperactivity or inattention, not both of them. In Tasi et al.’s study (17) with ICD-9-CM 314 a broader criterion was considered for ADHD diagnosis. Furthermore; selected exposure period was not the same among studies. Sprung considered this time before the age of two years (11). Tasi et al. (17) before 3 and in our study it was extended to before 4. The other noticeable factor was duration of follow-up periods. In Ko et al. (25) study children aged 5 - 10 years were focused on. Therefore cases diagnosed after 10 years of age could be missed. Due to different interpretations among observational studies and the multifactorial nature of the mentioned criteria, focusing on other modalities such as biomarkers and neuroimaging might provide more reliable results. There are still several unanswered questions: anesthetic drugs, doses, anesthesia duration, age at exposure and proper evaluation criteria. We acknowledge that to achieve more meaningful results, cohort studies with an adequate sample size is required. Surely, neuro behavioral disorders are multifactorial and similar to other supporting studies we cannot claim that we have found a single causative factor. However, despite the inconstant results of clinical studies and unanswered questions in this field, based on accumulating evidence suggesting irreversible neuronal damage and lasting neurodevelopmental sequels, it is wise to avoid any unnecessary procedure requiring GA in early life. Obviously children’s deprivation of anesthesia and analgesia is not legally or ethically accepted (29). Definitely to achieve the desired goals, not only anesthesiologists but also other specialists should be aware from the potential risks of GA administration during early life (15). Indeed proper communication with other involved physicians who refer the children for an elective surgery or invasive diagnostic procedure requiring GA which could be postponed is crucial (30). Providing sufficient knowledge in general society especially among parents should be considered as well. As such parents frequently question the physicians about the safety of GA in their children (31).
5.1. Limitations
We admit that there are several limitations for this work. Indeed due to the nature of this study, our data was achieved via a telephone interview and in many cases parents did not have a proper communication or might not remember the required data.
Therefore we were not able to infer causality either and clarify the pure effects of GA because it was hard to differentiate how the potential confounding factors such as maternal smoking, alcoholism, mental statue, child nutrition, lead exposure, child hood systemic and inflammatory diseases could affect the results.
5.2. Conclusions
This study showed that early exposure to GA might be a risk factor for later developing ADHD. Boys might be more sensitive to the long term adverse effects of anesthetic agents than girls. However, we believe that to confirm what is reported here and to determine the possible mechanisms for this association, future studies are required.