1. Context
1.1. Neuroleptic Malignant Syndrome Overview
Neuroleptic malignant syndrome (NMS) has been introduced as a rare and life-threatening side effect of antipsychotics. This complication was initially described by Delay et al. in 1968 due to haloperidol use (1, 2). The incidence rate of this side effect in patients taking psychotropic medications has been reported as 0.02% - 1.4%, with a mortality rate of 10% - 20% (3-5). The main symptoms of NMS resemble malignant hyperthermia, including increased body temperature (fever), imbalance of autonomic nervous system, and loss of consciousness. Moreover, NMS can lead to patient death through rhabdomyolysis or cardiovascular collapse. According to related reports in numerous studies, the incidence rate of NMS has witnessed a descending trend over recent years, associated with the growing use of atypical antipsychotics (6).
1.2. Drugs and Neuroleptic Malignant Syndrome
Although NMS has been historically considered a haloperidol side effect, a wide range of other psychotropic medications can be involved in this respect (7). Besides, NMS can arise as a side effect of drugs that block the main dopaminergic pathways, including metoclopramide, amoxapine, and lithium (1). Metoclopramide is also known as an anti-emesis medication that can bring about motor disorders such as excessive pyramidal reactions and tardive dyskinesia (8). The NMS development following metoclopramide consumption is rare even though dopamine blockage after the intake of this medication has already been proven (9, 10).
1.3. Neuroleptic Malignant Syndrome Symptoms
The neuroleptic malignant syndrome has been introduced as a rare but lethal and idiosyncratic reaction to neuroleptics/antipsychotics (5, 9). In uncommon cases, generally associated with low-potency antipsychotics, muscle rigidity may be milder or may not be observed at all. Furthermore, an increase in body temperature, as one of the most essential signs for NMS diagnosis, may not be reported in such cases. If there is only one symptom of NMS, such as autonomic dysfunction, high body temperature (fever), increased creatine kinase (CK), or muscle rigidity like that occurring in Parkinson’s disease, diagnosis can be very difficult because none of these symptoms alone suggests NMS (10).
Leukocytosis is another symptom of this syndrome. Like other drug-induced reactions, NMS should be considered after ruling out other diagnoses, including meningitis, malignant hypertrophy, tumor, viral encephalitis, seizure, acute lethal catatonia, hyperthyroidism, and poisoning with anticholinergic drugs (11).
1.4. Neuroleptic Malignant Syndrome Etiology
The exact cause of NMS development is unknown. However, NMS can be assumed as the result of maladjustments in several neuromuscular and intrinsic nervous systems occurring at the last stage of the hypermetabolic syndrome (5). Hypermetabolic muscle state is also one of the two main theories raised about the pathogenesis of this syndrome (similar to what appears in malignant hyperthermia). For this reason, muscle relaxants such as dantrolene sodium have been effective in some cases for reducing muscle rigidity and fever. The second theory in this domain is that NMS is a consequence of reduced diphtheria activity in the central nervous system (12).
It should be noted that there are three main dopaminergic activity pathways, including (A) nigrostriatal; (B) mesolimbic/cortical; and (C) hypothalamic. It seems that acute obstruction of nigrostriatal and hypothalamic dopamine activity pathways can trigger the main symptoms of NMS. This blockage can also be made in two ways: (A) D2 dopamine receptor obstruction after taking neuroleptic drugs and disorder in core body temperature regulation and muscle tone due to disrupted dopaminergic pathways; accordingly, it is recommended to use dopamine agonists such as bromocriptine for NMS treatment. Reports of NMS development following the sudden withdrawal of levodopa also support this hypothesis (9, 12); (B) independent depletion in dopamine activity and its direct effects on skeletal muscles in an environment that can correspondingly play a double role (12).
There is strong evidence that genetic defects are considered important risk factors (13). The onset of NMS as a side effect following drug intake also varies, and most cases are instigated within the first two weeks (14). In terms of gender, men (especially those aged below 40 years) are at higher risks than women (ale to fame ratio 2:1). However, women experiencing a post-partum period are at a higher risk of developing NMS. because of the possibility of being affected by psychiatric disorders and taking neuroleptic medications. The most obvious risk of NMS is the use of neuroleptics, especially highly potent ones (10). The higher the number of the drugs and the higher the dosage (using effective or average effective doses) and the faster the increased medication dose (increased dosage within five days of starting the medication), the higher the risk of developing this syndrome. Thus, a previous history of NMS, a recent episode of catatonia, and severe agitation are all taken into account as risk factors in this domain (9, 10). The onset of NMS is generally progressive, and its symptoms develop slowly over two to three days; however, its fulminant form is possible with a sudden onset of a few hours. The neuroleptic malignant syndrome may be simply confused with dystonic reactions that are more common and less risky. These reactions are also more localized and respond rapidly to intravenous drugs such as benzatropine (14).
1.5. Neuroleptic Malignant Syndrome and Metoclopramide
Metoclopramide is known as an anti-nausea medication administered before surgery to manage digestive problems. It also increases the tone of the esophageal wall and is utilized as prophylaxis for pulmonary aspiration (15). Besides, metoclopramide is used in combination with chemotherapy medications for cancer patients and the control of diabetic gastropathy. Previously, it seemed that metoclopramide was a chlorobenzamide and not in the phenothiazine group but free of extrapyramidal side effects. Sequential reports of complications such as acute dysplasia, Parkinsonism, and delayed dystonia also indicate that metoclopramide can bring about extrapyramidal symptoms and drug-induced motor side effects (15, 16). Although metoclopramide causes ocular and pyramidal complications and NMS as a worse form, it is being widely used in clinical practice (17). The onset of this side effect varies following metoclopramide intake, although it occurs more commonly within the first two weeks of consumption.
Additionally, NMS can persist for days after drug intake. This complication can also be seen at the usual dose of 10 mg every six hours (40 mg per day) and may even occur following the first dose (14). In this study, the related literature about metoclopramide-induced NMS was reviewed using a narrative method.
2. Evidence Acquisition
2.1. Research Question
A review of the related literature was performed using a narrative method. The research question addressed in this study was as follows:
“What are the factors affecting NMS development following metoclopramide intake, and how can this syndrome be managed?”
2.2. Search on Databases
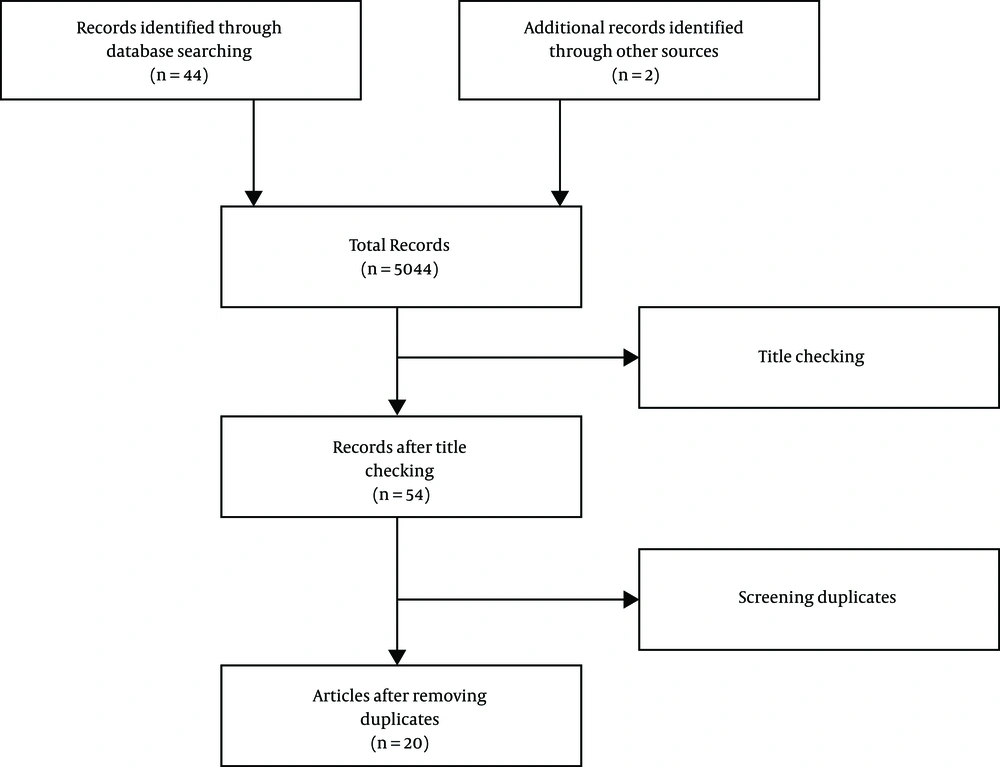
After raising the question, a comprehensive search was conducted for relevant resources in English by two researchers independently in the databases including PubMed, Google Scholar, and ScienceDirect using the keywords “metoclopramide” and “neuroleptic malignant syndrome”. In this respect, the total number of 5,044 articles were retrieved. Furthermore, there was a separate search for relevant articles in their reference sections, and finally, two additional articles were added.
2.3. Article Selection
A total number of 5,046 articles were obtained after the initial search. Then, the two researchers independently screened out their titles and abstracts, and the related ones were selected based on the inclusion criteria: (A) articles published in scientific journals and (B) articles addressing the research question. The screening resulted in the removal of 5,002 articles, and finally, 54 articles remained. After the exclusion of duplicate articles, 24 articles were confirmed (Figure 1). Ultimately, 20 articles were evaluated using the CARE Checklist (Appendix 1 in Supplementary File). The scores of the articles are illustrated in Table 1.
| CARE | Study | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3a, b, cb | 4 | 5 | 6a, b, c | 7 | 8a, b, c, d | 9a, b, c, d | 10a, b, c | 11a, b, c, d | 12 | 13 | 14 | Score | |
| Robinson et al. (18) | - | - | 1+ | + | - | 3+ | + | 1+ | 4+ | - | 1+ | + | - | - | 12 |
| Samie (19) | + | - | - | + | - | 3+ | + | 3+ | 4+ | 3+ | 3+ | + | - | + | 21 |
| Friedman et al. (16) | - | - | 2+ | + | - | 3+ | + | 2+ | 2+ | 1+ | 1+ | + | - | - | 14 |
| Cassidy et al. (20) | - | - | 1+ | + | + | 3+ | + | 2+ | 2+ | 1+ | 1+ | + | - | - | 14 |
| Brower et al. (21) | - | - | 1+ | + | - | 3+ | + | 3+ | 4+ | 1+ | 1+ | + | - | - | 17 |
| Donnet et al. (12) | - | - | 1+ | + | - | 3+ | + | 1+ | 2+ | 1+ | 1+ | + | - | - | 12 |
| Henderson et al. (14) | - | + | 1+ | + | - | 3+ | + | 1+ | 1+ | 2+ | 2+ | + | - | - | 14 |
| Bakri et al. (22) | + | - | 1+ | + | - | 3+ | + | 2+ | 2+ | 1+ | 1+ | + | - | - | 14 |
| Fujita et al. (23) | - | - | 1+ | + | - | 3+ | + | 2+ | 2+ | - | 1+ | + | - | - | 12 |
| Shintani et al. (24) | - | - | 1+ | + | - | 3+ | + | 3+ | 2+ | 2+ | 1+ | + | - | - | 15 |
| Le Couteur and Kay (25) | - | - | 1+ | + | - | 3+ | + | 2+ | 2+ | 2+ | 1+ | + | - | - | 14 |
| Nonino et al. (26) | - | - | 2+ | + | - | 3+ | + | 2+ | 2+ | 2+ | 1+ | + | - | - | 15 |
| Nachreiner et al. (7) | - | - | 3+ | + | - | 3+ | + | 2+ | 4+ | 1+ | 2+ | + | - | - | 18 |
| Stein et al. (27) | - | - | 2+ | + | - | 2+ | + | 2+ | 2+ | - | 2+ | + | - | - | 13 |
| Supriwala et al. (28) | - | - | 3+ | + | - | 3+ | + | 3+ | 2+ | - | 2+ | + | - | - | 16 |
| Yaman et al. (1) | - | - | 3+ | + | - | 3+ | + | 2+ | 3+ | 1+ | 3+ | + | - | - | 18 |
| Mazhar et al. (11) | + | - | 3+ | + | - | 3+ | + | 2+ | 3+ | 2+ | 2+ | + | - | - | 19 |
| Wittman et al. (29) | + | + | 3+ | + | - | 3+ | + | 3+ | 4+ | 2+ | 3+ | + | - | - | 23 |
| Breeden et al. (30) | - | - | 1+ | + | - | 3+ | + | 2+ | 2+ | 2+ | 1+ | + | - | - | 14 |
| Kocyigit et al. (31) | - | - | 1+ | + | + | 3+ | + | 3+ | 3+ | 1+ | 1+ | + | - | + | 16 |
aBased on the CARE questionnaire for article quality assessment.
bTotal scores of rows with multiple points.
2.4. Ethical Considerations
Ethical issues and general standards for plagiarism, misconduct, reproductions or counterfeiting, simultaneous submissions or duplicate publications, and redundancy were observed in this review study.
2.5. Data Extraction
The full-texts of the final articles were carefully studied. It should be noted that access to the full-texts of four articles was not possible due to the non-English language of those articles. Therefore, necessary and relevant information was extracted from 20 articles and classified as shown in Table 2.
| Type of Study | Patient’s Age, y | Patient’s Gender | Causes of Metoclopramide Administration | Metoclopramide Dose/Administration Type | Main Symptoms of NMS | Treatment | Results | |
|---|---|---|---|---|---|---|---|---|
| Robinson et al. (18) | Case report | 53 | Male | Nausea and vomiting after coughing and fever | 20 mg/muscular | Ververi-Brady syndrome, difficulty in swallowing, talking, and breathing, muscle rigidity, sweating | Anticholinergics and levodopa (2 g per day) | Successful treatment |
| Samie (19) | Case report | 68 | Male | Nausea | 10 mg every hour/intravenous | Fever, loss of consciousness, pain in the upper and lower extremities, slow motion and talk | Bromocriptine (5 mg three times a day) | Patient’s death |
| Friedman et al. (16) | Case report | 36 | Female | Nausea during azotemia | 10 mg every six hours/intravenous | Fever, loss of consciousness, catatonia | Lorazepam (2 mg intravenous) and bromocriptine (2.5 mg three times a day) | Successful treatment |
| Cassidy et al. (20) | Case report | 73 | Male | Nausea | 30 mg/oral | Disorientation, diffuse muscle rigidity, fever | Bromocriptine (5 mg three times a day) | Patient’s death |
| Brower et al. (21) | Case report | 5 | Male | Prophylactic chemotherapy treatment | 0.5 mg/kg/intravenous | Restlessness, diffuse muscle rigidity, dysentery, fever | Dantrolene (20 mg per day) | Successful treatment |
| Donnet et al. (12) | Case report | 68 | - | Vomiting | 10 mg | Lethargy, diffuse muscle rigidity, fever | Dantrolene (2 mg/kg/tid) | Patient’s death |
| Henderson et al. (14) | Case report | 70 | Male | Stimulated gastrointestinal motility | 10 mg/ intravenous | Diffuse muscle rigidity, immobilization, fever | Benztropine and dantrolene (2 mg/kg) | Successful treatment |
| Bakri et al. (22) | Case report | 50 | Female | Nausea and vomiting after surgery | 80 mg | Dizziness, restlessness, sweating, diffuse muscle rigidity | Dantrolene (2 mg/kg/day) | Successful treatment |
| Fujita et al. (23) | Case report | 54 | Female | - | 15 mg/oral | Coma, muscle cramps, fever | Dantrolene | Successful treatment |
| Shintani et al. (24) | Case report | 77 | - | Gastroparesis | 20 mg/oral | Immobilization, impaired swallowing and talking, drooling | Carbidopa levodopa (300 mg), amantadine (200 mg), and Bromocriptine (7.5 mg) | Successful treatment |
| Le Couteur and Kay (25) | Case report | 62 | Male | Nausea | 20 - 40 mg | Fever, urinary incontinence, loss of consciousness | Bromocriptine (7.5 mg twice a day) | Successful treatment |
| Nonino et al. (26) | Case report | 80 | Female | Nausea | 10 mg twice daily | Loss of consciousness, fever, diffuse muscle rigidity, coma | Bromocriptine (2.5 mg three times a day) | Patient’s death |
| Nachreiner et al. (7) | Case report | 27 | Male | Enhanced oral tolerance | 10 mg Every six hours | Arrhythmia, blurred urine, fever | Dantrolene (2 mg/kg boluses) and (10 mg/kg/day) | Successful treatment |
| Stein et al. (27) | Case report | 6 | Female | Vomiting | - | Fever, irritability, skin discoloration, muscle rigidity, increased heart rate, hypotension | Injectable dantrolene | Successful treatment |
| Supariwala et al. (28) | Case report | 58 | Male | Gastroparesis | 10 mg twice daily | Dizziness, dysarthria, lethargy, mild muscle rigidity | Dantrolene IV (60 mg boluses) and (25 mg q8h), bromocriptine (2.5 mg q6h), and cyproheptadine (2 mg q8h) | Successful treatment |
| Yaman et al. (1) | Case report | 2 | Female | Vomiting | 10 mg/muscular | Fever, muscle spasm, loss of consciousness | Biperiden (0.04 mg/kg) and diphenhydramine (5 mg/kg/day) | Successful treatment |
| Mazhar et al. (11) | Case report | 64 | Male | Nausea | 10 mg every 6 hours/intravenous | Dizziness, sweating, dyspnea, muscle rigidity | Dantrolene (1 mg/kg-stat), carbidopa (25 mg), levodopa (100 mg orally), and baclofen (5 mg orally) | Successful treatment |
| Wittman et al. (29) | Case report | 13 | Male | Vomiting | 10 mg three times daily | Blindness, hypotension, muscle rigidity | Intravenous dopamine (infusion) (10 mg/kg/min) | Successful treatment |
| Breeden et al. (30) | Case report | 84 | Female | Gastroparesis | 10 mg every eight hours | Hypertension, increased heart rate, muscle rigidity, fever | Diphenhydramine (50 mg intravenous), lorazepam (2 mg), and Midazolam (4 mg) | Successful treatment |
| Kocyigit et al. (31) | Case report | 74 | Male | Nausea and vomiting | 10 mg one dose/intravenous | Loss of consciousness, muscle rigidity, disorientation, fever, muscle rigidity | Bromocriptine (2.5 mg q6h) and lorazepam (0.5 mg q8h) | Successful treatment |
3. Results
Although NMS following metoclopramide intake is reported very rarely, this medication is being widely used and even one dose can be as much as necessary to initiate this syndrome. Therefore, NMS should be considered for any psychiatric disorders with unexpected mental changes, muscle rigidity, and fever after being treated with metoclopramide (16).
Of the 20 articles reporting metoclopramide-induced NMS, treatments were successful in 16 cases (80%), but it led to the death of four patients (20%). Deaths were due to severe respiratory distress with cyanosis and hypotension (19), bilateral bronchopneumonia with extensive left frontoparietal lobe infarction (20), the sudden dropping of blood pressure, and coma (12), and diffuse brain edema (26). There were 11 and eight male and female patients, respectively, and gender was not mentioned for a patient in one article. The age range of the patients was from six months to 84 years, and the mean age was 50.92 years.
Although most cases of NMS occurred following multiple doses of metoclopramide intake, in a 74-year-old patient reported by Kocyigit et al. (31), NMS was developed only after one dose of metoclopramide (10 mg), and the patient recovered following appropriate diagnosis and treatment with bromocriptine. Attributable to the multiplicity of the complications, the diagnosis of this syndrome in patients with a higher age is difficult. Therefore, a decrease in connectivity to dopamine receptors via dopamine transporter single-photon emission computed tomography or metaiodobenzylguanidine scintigraphy could aid in diagnosis (31). Although the NMS incidence following the intake of this drug is rare, it is of common use, and it should be deemed that the given syndrome may develop even following one dose. Consequently, NMS should be taken into account in all patients treated with metoclopramide who are subjected to unacceptable changes in consciousness, muscle rigidity, and fever (29).
In a case report by Fujita et al. (23) in 1995, a 54-year-old woman was introduced with kidney failure undergoing hemodialysis (three times a week). The patient had been treated with various medications, including oral metoclopramide (15 mg daily). At the beginning of one of the hemodialysis sessions, the patient was reported to be agitated and confused and suffered from the coma, cramped organs, and fever at the end of the session. Ultimately, the patient was treated with dantrolene following NMS diagnosis, and a significant improvement was observed in fever and muscle rigidity after the first dosage. This report and the review of the related literature (four similar cases) suggest that metoclopramide intake in patients affected with kidney failure may be accompanied by a higher risk of NMS. Besides, a pharmacokinetic study reported that metoclopramide is likely to accumulate in patients with renal insufficiency or those undergoing hemodialysis, as the renal clearance of metoclopramide reduces or its removal is inadequate in hemodialysis. This case and other similar ones demonstrate that metoclopramide dosage should be adjusted in patients with kidney failure and a great deal of attention should be drawn to the possibility of NMS development (23).
In a case report in 1987, Samie (19) observed NMS in a patient with renal dysfunction following metoclopramide intake, suggesting that metoclopramide dosage adjustment is of utmost importance in patients affected with kidney failure since drug clearance depends on the adequacy of renal functioning. In patients with chronic renal failure, a dose reduction of up to 60% was recommended. Although NMS appears to be an idiosyncratic reaction, these symptoms may be due to high levels of metoclopramide serum accumulation following kidney failure (19).
Similarly, in a study by Friedman et al. (16), two diabetic patients affected with neuropathy, retinopathy, and nephropathy accompanied by the symptoms of fever, leukocytosis, and altered consciousness were diagnosed with NMS. Both patients had a history of occasional metoclopramide intake for diabetic gastrectomy treatment. Both of them were also suffering from kidney failure. One patient required to restart metoclopramide to control severe gastrectomy. For this reason, bromocriptine was initiated as a prophylactic agent to restart the medication. Interestingly, the re-use of metoclopramide did not lead to permanent NMS recurrence, and prophylaxis was not necessary for it (16). In addition, the sudden onset of fever, changes in vital signs, variations in consciousness, muscle rigidity, and the main symptoms of NMS are expected to be considered in the domain of care for elderly patients treated with gastrointestinal medications, especially anti-nausea ones (11).
In a case report by Stein et al. (27), NMS was diagnosed following metoclopramide intake in a six-month infant suffering from Freeman-Sheldon syndrome, which could cause multiple muscle contractions and pharyngeal abnormalities as a congenital myopathy. Patients with this disorder were also at risk of NMS development that could overlap malignant hyperthyroidism. In terms of the patient’s age, this case report of NMS was unique among all the 20 articles, as it evaluated NMS following metoclopramide intake. This study underlined the need to pay much more attention to NMS in febrile children, patients with underlying musculoskeletal disorders, and those treated with anti-nausea medications and dopamine antagonists. Even though the causes of infectious diseases could not be definitively determined in the introduced case, this study suggested hyperthermia in this infant, accompanied by other symptoms including a sudden increase in temperature, generalized muscle rigidity, autonomic symptoms, acidosis, hypoxia, and rhabdomyolysis, which could all meet the NMS criteria (27). Therefore, the higher the number of medications and the higher the dosage (using effective or average effective doses) and the faster the increased drug dosage (increasing the dosage within five days of starting the medication), the higher the risks of developing this syndrome. Accordingly, a previous history of NMS, a recent episode of catatonia, and severe agitation are all taken into account as risk factors in this domain (9, 10).
3.1. Neuroleptic Malignant Syndrome General Management and Clinical Outcomes
There is strong evidence that genetic defects comprise an important risk factor (13). The onset of this complication is also different from the start of the drug intake, but most cases occur within the first two weeks of consumption (14). In terms of gender, men (especially those aged below 40 years) were at higher risks than women (ale to fame ratio 2:1). However, women experiencing a post-partum period are at a higher risk of NMS development owing to the possibility of acquiring psychiatric disorders and taking neuroleptics. The most obvious risk factor for NMS development was the intake of neuroleptic medications, especially highly potent ones (10). Therefore, the higher the number of the drugs and the higher the dosage (using effective or average effective doses) and the faster the increased drug dosage (increasing the dosage within five days of starting the medication), the higher the risks of developing this syndrome. A previous history of NMS, a recent episode of catatonia, and severe agitation were all regarded as risk factors (9, 10).
Since NMS has a different onset and severity, choosing effective treatments for this syndrome is very complicated. The history of each episode is not also the same, and the interval between symptom onset, diagnosis, and treatment varies from one patient to another. In addition, medications accepted to treat this syndrome have been used with different doses during treatments, both as a single drug and in combination with other ones (30). Therefore, this phenomenon is not very so often diagnosed, less likely to be detected, and not even adequately treated. It should be noted that diagnosis and treatment are vital in the early stages of the disease (6). Among the essential components of NMS management is the discontinuation of the causative drug, along with supportive care therapies, such as cooling patients and transferring fluid to them (30). According to the results of related studies in this domain, it has been recommended to discontinue causative agents without definite diagnoses, even if there is an atypical manifestation of NMS. Reducing the risk of mortality and morbidity associated with this syndrome outweighs the risk of not treating the underlying disease or agitation of the patient following abrupt discontinuation of the drug (31). In this respect, the oral administration of dopamine agonists such as bromocriptine, amantadine, and levodopa can be effective. Moreover, dantrolene has been positive in treating this syndrome, leading to dramatic and immediate reductions in muscle rigidity and body temperature. The benefits of dantrolene are probably related to its muscle relaxant effects (14, 17). It should be noted that drug treatment is not helpful in all cases. If several treatments fail, electroconvulsive therapy may be useful, and it is recommended for patients who are relatively responding to medication-based treatments to eliminate the remaining symptoms (31). Although studies have reported significant reactions of NMS to treatments, mortality rates following this syndrome are high (32). Furthermore, the prognosis can be different depending on complications (rhabdomyolysis, kidney failure, aspiration pneumonia, sepsis, and pulmonary embolism). The mortality rate in the case of early diagnosis and treatment of NMS is 5% - 20%, but it can reach 70% in the event of side effects (33, 34).
It is not easy to exclude the confounding factors, including different types of medications simultaneously taken by patients or even other underlying causes for the symptoms reported. Therefore, reaching a conclusion based on case reports with their weaknesses would be hard. Accordingly, further investigations are required to examine the precise relationship between metoclopramide use and NMS occurrence.
4. Conclusions
Although neuroleptic malignant syndrome following metoclopramide intake is reported very rarely, it should be considered for any psychiatric symptoms with unexpected mental changes, muscle rigidity, high blood pressure, diaphoresis, and fever after treatment with this medication. The neuroleptic malignant syndrome can similarly occur following multiple doses or just one dose of metoclopramide intake. Moreover, metoclopramide use in patients affected with kidney failure can be accompanied by a higher risk of NMS. Accordingly, a previous history of NMS, recent catatonic episodes, and severe agitation are all taken into account as risk factors in this domain. Since NMS has a different onset and severity, choosing effective treatments for this life-threatening neurologic emergency is complicated. The history of each episode is not also the same, and the interval between symptom onset, diagnosis, and treatment varies from one patient to another.